| Identification | More | [Name]
Cesium bromide | [CAS]
7787-69-1 | [Synonyms]
CESIUM BROMIDE
Caesium bromide
Caesiumbromide
Cesium bromide (CsBr)
cesiumbromide(csbr)
CsBr
Tricesium tribromide
tricesiumtribromide
Caesiumbromid
Cesium bromide (99.9% Cs)
Cesium bromide,(99% Cs)
Cesiumbromidewhitextl
CESIUM BROMIDE, 99.999%
CESIUM BROMIDE, 99.9% METALS BASIS
CESIUM BROMIDE, ANHYDROUS, BEADS,-10 ME SH, 99.9%
CESIUM BROMIDE 99%
CESIUM BROMIDE, ANHYDROUS, 99.999%
CaesiumBromideA.R.
Cesiumbromide,Puratronic,99.998%(metalsbasis)
Cesiumbromide,99.999%(metalsbasis) | [EINECS(EC#)]
232-130-0 | [Molecular Formula]
BrCs | [MDL Number]
MFCD00010954 | [Molecular Weight]
212.81 | [MOL File]
7787-69-1.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
FK9275000
| [TSCA ]
Yes | [HS Code ]
28275900 | [Safety Profile]
Moderately toxic by
intraperitoneal route. See also CESIUM and
BROMIDES. When heated to
decomposition it emits toxic fumes of Br-. | [Toxicity]
LD50 i.p. in rats: 1.4 g/kg (Cochran) |
| Questions And Answer | Back Directory | [Structure]
Cesium bromide (CsBr) is Tetraauricupride structured and crystallizes in the cubic Pm?3m space group. Cs1? is bonded in a body-centred cubic geometry to eight equivalent Br1? atoms. All Cs-Br bond lengths are 3.75 ?. Br1? is bonded in a body-centered cubic geometry to eight equivalent Cs1? atoms.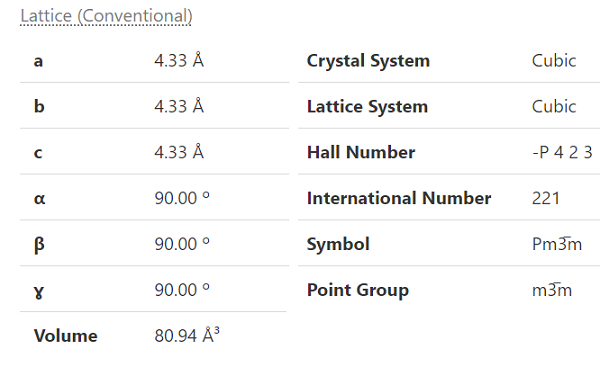 |
| Hazard Information | Back Directory | [Description]
Cesium bromide is a colorless crystal with the formula CsBr. It is a moderately water soluble crystalline Cesium source that decomposes to Cesium oxide on heating. Most metal bromine compounds are water-soluble and are used in water treatment, chemical analysis, and ultra-high purity for certain crystal growth applications. | [Chemical Properties]
Cesium bromide is a colourless crystals. It has been used in protein crystallization for anamolous scattering.

| [Uses]
Cesium bromide (CsBr) crystals are used in scintillation counters to detect radiation. The
compound is also used to coat the inside of fluorescent screens. | [Uses]
Cesium bromide is used as reagent, intermediate in pharmaceuticals. | [Uses]
In x-ray fluorescent screens, spectrometer prisms, absorption-cell windows. | [Preparation]
Caesium bromide can be prepared via following reactions:
Neutralization:
CsOH (aq) + HBr (aq) → CsBr (aq) + H2O (l)
Cs2(CO3) (aq) + 2 HBr (aq) → 2 CsBr (aq) + H2O (l) + CO2 (g)
Direct synthesis:
2 Cs (s) + Br2 (g) → 2 CsBr (s)
The direct synthesis is a vigorous reaction of caesium with other halogens. Due to its high cost, it is not used for preparation. | [Production Methods]
Single crystals Cesium bromide can be synthesized by the Kyropoulus method or the Stockbarger method. One can polish the crystal using pure alcohol.
| [Purification Methods]
It is very soluble in H2O, soluble in EtOH but insoluble in Me2CO. Dissolve it in the minimum volume of H2O, filter and precipitate it by adding Me2CO. Filter off the solid and dry it at 100o. Also recrystallise it from water (0.8mL/g) by partial evaporation in a desiccator. | [Structure and conformation]
The space lattice of CsBr(Cesium bromide) belongs to the cubic system, and its cesium chloride structure has a lattice constant of a=0.429 nm.
|
|
|