| Identification | More | [Name]
Trimethoprim | [CAS]
738-70-5 | [Synonyms]
2,4-DIAMINO-5-(3,4,5-TRIMETHOXYBENZYL)PYRIMIDE
2,4-DIAMINO-5-(3',4',5'-TRIMETHOXYBENZYL)PYRIMIDINE
2,4-DIAMINO-5-(3,4,5-TRIMETHOXYBENZYL)PYRIMIDINE
5-(3,4,5-trimethoxybenzyl)-2,4-diaminopyrimidine
5-(3,4,5-TRIMETHOXYBENZYL)PYRIMIDINE-2,4-DIYLAMINE
5-((3,4,5-trimethoxyphenyl)methyl)-2,4-pyrimidinediamine
abaprim
TMP
TRIMETHOPRIM
TRIMETHORPIM
TRIMETOPRIM
2,4-diamino-5-(3,4,5-trimethoxybenzyl)-pyrimidin
2,4-Pyrimidinediamine, 5-[(3,4,5-trimethoxyphenyl)methyl]-
5-((3,4,5-trimethoxyphenyl)-methyl)-4-pyrimidinediamine
5-(3,4,5-Trimethoxybenzyl)-2,4-pyrimidinediamine
Bactramin
BW 56-72
bw56-72
component of Bactrim
Instalac | [EINECS(EC#)]
212-006-2 | [Molecular Formula]
C14H18N4O3 | [MDL Number]
MFCD00036761 | [Molecular Weight]
290.32 | [MOL File]
738-70-5.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline | [Melting point ]
199-203 °C | [Boiling point ]
432.41°C (rough estimate) | [density ]
1.1648 (rough estimate) | [refractive index ]
1.6000 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
DMSO: soluble
| [form ]
white powder
| [pka]
6.6(at 25℃) | [color ]
colorless or white | [Stability:]
Stable. Incompatible with strong oxidizing agents, acids. | [biological source]
synthetic | [Water Solubility ]
<0.1 g/100 mL at 24 ºC | [Usage]
Antibacterial. | [Merck ]
9709 | [BRN ]
625127 | [BCS Class]
2 | [CAS DataBase Reference]
738-70-5(CAS DataBase Reference) | [NIST Chemistry Reference]
Trimethoprim(738-70-5) | [EPA Substance Registry System]
738-70-5(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R25:Toxic if swallowed. | [Safety Statements ]
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
3249 | [WGK Germany ]
3
| [RTECS ]
UV8225000
| [F ]
8-10-21 | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29335900 | [Hazardous Substances Data]
738-70-5(Hazardous Substances Data) | [Toxicity]
LD50 orally in mice: 7000 mg/kg (Yamamoto) |
| Hazard Information | Back Directory | [General Description]
Odorless white powder. Bitter taste. | [Reactivity Profile]
This compound readily forms salts with acids. . | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available. TRIMETHOPRIM is probably combustible. | [Description]
Trimethoprim selectivity between bacterial and mammalian
dihydrofolate reductases results from the subtle but significant architectural differences between these
enzyme systems. Whereas the bacterial enzyme and the mammalian enzyme both efficiently catalyze the
conversion of dihydrofolic acid to tetrahydrofolic acid, the bacterial enzyme is sensitive to inhibition by
trimethoprim by up to 40,000-fold lower concentrations than the mouse enzyme is. This difference explains
the useful selective toxicity of trimethoprim. | [Originator]
Eusaprim,Wellcome,Italy,1970 | [Definition]
ChEBI: Trimethoprim is an aminopyrimidine antibiotic whose structure consists of pyrimidine 2,4-diamine and 1,2,3-trimethoxybenzene moieties linked by a methylene bridge. It has a role as an EC 1.5.1.3 (dihydrofolate reductase) inhibitor, a xenobiotic, an environmental contaminant, a drug allergen, an antibacterial drug and a diuretic. It is a member of methoxybenzenes and an aminopyrimidine. | [Manufacturing Process]
6 grams (0.26 mol) sodium was dissolved in 300 ml methanol under stirring
and refluxing. 47.5 grams (0.55 mol) β-methoxypropionitrile and 98 grams
(0.5 mol) 3,4,5-trimethoxybenzaldehyde were added and the mixture refluxed
gently for 4 hours. The mixture was then chilled and 150 ml of water was
added. The product crystallized rapidly. Crystallization was allowed to proceed
at 5° to 10°C under stirring for 1 hour. The product was filtered by suction
and washed on the filter with 200 ml of 60% ice cold methanol. The crude
material was air-dried and used for further steps without purification. It
melted at 78° to 80°C. A pure sample, recrystallized from methanol, melted
at 82°C. The yield of 3,4,5-trimethoxy-2'-methoxymethylcinnamonitrile was
92 grams, corresponding to 70% of the theory.
19 grams (0.83 mol) sodium was dissolved in 300 ml methanol, 106 grams of
3,4,5-trimethoxy-2'-methoxymethylcinnamonitrile was added and the mixture
gently refluxed for 24 hours. The solution, which had turned brown, was
poured into 1 liter of water and the precipitated oil extracted repeatedly with
benzene. The combined benzene layers (500 to 700 ml) were washed 3 times
with 500 ml of water, the benzene removed by evaporation in a vacuum from
a water bath, and the brown residual oil distilled in vacuo, boiling point 215°
to 225°C/11 mm. The clear, viscous oil, 3,4,5-trimethoxy-2'-cyano_x0002_dihydrocinnamaldehyde dimethyl acetal, weighed 83 grams (71% of the
theory), and showed a nD23 = 1.5230. It solidified upon standing. A sample
recrystallized from methanol melted at 69° to 70°C and showed a strong
melting point depression with the starting material; nD25 = 1.5190.
31.5 grams (0.107 mol) 3,4,5-trimethoxy-2'-cyano-dihydrocinnamaldehyde
dimethyl acetal was refluxed with methanolic guanidine solution (200 ml
containing 0.25 mol of guanidine) for 2 hours. The methanol completely
distilled off under stirring, finally from a bath of 110° to 120°C until the
residue solidified completely to a yellowish crystalline mass. After allowing to
cool, it was slurried with 100 ml of water and collected by vacuum filtration
and dried. The yield of 2,4-diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine
amounted to 28 grams (91% of the theory). The material showed the correct
melting point of 199° to 200°C and was, however, yellowish discolored.
20 grams of the above product was added to 30 ml of 3 N aqueous sulfuric
acid at 60°C under stirring. The solution was chilled under stirring to 5° to
10°C. The crystalline sulfate was collected by vacuum filtration and washed on the filter twice with 10 ml of cold 3 N aqueous sulfuric acid each time. From
the filtrate there was recovered 1.3 grams (6.5%) of discolored material
melting at 195° to 196°C and which can be added to subsequent purification
batches.
The sulfate on the filter was dissolved in 200 ml of hot water, the solution
charcoaled hot, and the product precipitated from the clear colorless filtrate
by the gradual addition of a solution of 20 grams of sodium hydroxide in 40
ml of water under chilling. The precipitate was filtered by suction and washed
thoroughly with water on the filter. The white material, 17.5 grams (88%)
showed the correct melting point of 200° to 201°C, according to US Patent
3,341,541. | [Brand name]
Proloprim (Monarch); Trimpex
(Roche). | [Therapeutic Function]
Antibacterial (urinary) | [Antimicrobial activity]
Trimethoprim has a broad spectrum of antimicrobial activity. It is 20–100 times more
active than sulfamethoxazole with respect to most bacterial forms. Trimethoprim is active
with respect to Gram-positive, aerobic bacteria such as Staphylococcus aureus,
Staphylococcus epidermidis, and various types of Streptococcus and Listeria monocyto�genes. Trimethoprim is inferior to sulfonamides against forms of Nocardia. It is active with respect to Gram-negative, aerobic bacteria such as most E. coli, Enterobacter,
Proteus, Klebsiella, Providencia, Morganella, Serratia marcescens, Citrobacter,
Salmonella, Shigella, Yersinia enterocolitica that are sensitive to trimethoprim.
Trimethoprim is also active with respect to Legionella, Acinetobacter, Vibrio,
Aeromonas, Pseudomonas maltophila, P. cepacia, although P. aeruginosa is resistant to
trimethoprim. | [Biochem/physiol Actions]
Inhibits the synthesis of tetrahydrofolate by procaryote specific dihydrofolate reductase (DHFR). | [Mechanism of action]
Haemophilus influenzae and H. ducreyi are sensitive to trimethoprim. Pathogenic
Neisseria (meningococci and gonococci) and Branhamella catarrhalis are moderately
resistant to trimethoprim, although they are very sensitive to a combination of trimetho�prim and sulfamethoxazole. Anaerobic bacteria in general are resistant to trimethoprim,
although a combination of trimethoprim-sulfamethoxazole does have an effect on them.
Pneumocystis carinii is also sensitive to that combination.
Bacterial resistance to trimethoprim can originate because of a number of reasons:
inability of the drug to penetrate through the membrane (P. aeruginosa); the presence of
dihydrofolate reductase that is not sensitive to inhibition by trimethoprim; overproduction
of dihydrofolate reductase and mutation expressed as thyminic dependence, when the
organism requires exogenic thymine for synthesizing DNA, i.e. bypassing metabolic
blockage caused by trimethoprim.
Resistance to a combination of trimethoprim-sulfamethoxazole is always less frequent
than when any of these drugs is used separately. This combination of drugs, which is
known by the commercial names cotrimoxazole, bactrim, biseptol, sulfatrim, and many
others, is used for treating infections of the respiratory tract, infections of the urinary tract,
gastric infections, surgical infections, enteritis, meningitis, and other diseases. | [Clinical Use]
Trimethoprim (5-[(3,4,5-trimethoxyphenyl)methyl]-2,4-pyrimidinediamine or 2,4-diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine) is closely related to several antimalarialsbut does not have good antimalarial activity by itself; it is,however, a potent antibacterial. Originally introduced incombination with sulfamethoxazole, it is now available as asingle agent.
Approved by the FDA in 1980, trimethoprim as a singleagent is used only for the treatment of uncomplicatedurinary tract infections. The argument for trimethoprim asa single agent was summarized in 1979 by Wormser andDeutsch. They point out that several studies comparingtrimethoprim with TMP–SMX for the treatment ofchronic urinary tract infections found no statistically relevantdifference between the two courses of therapy.The concern is that when used as a single agent, bacterianow susceptible to trimethoprim will rapidly developresistance. In combination with a sulfonamide, however,the bacteria will be less likely to do so. That is, they willnot survive long enough to easily develop resistance toboth drugs. | [Synthesis]
Trimethoprim, 2,4-diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine (33.1. 51), is synthesized in various ways. The first scheme of synthesis begins with ethyl ester of 3,4,5-trimethoxydehydrocinnamic acid, which is formylated with ethyl formate using sodium as a base to make an enol of the semialdehyde 3,4,5-trimethoxybenzylmalonic ester (33.1.49), which undergoes a heterocyclization reaction with guanidine to make 2-amino- 4-hydroxy-5-(3,4,5-trimethoxybenzyl)pyrimidine (33.1.50). Subsequent replacement of the hydroxyl group in the resulting product with chlorine using phosphorous oxychloride and then with an amino group using ammonia gives the desired trimethoprim.
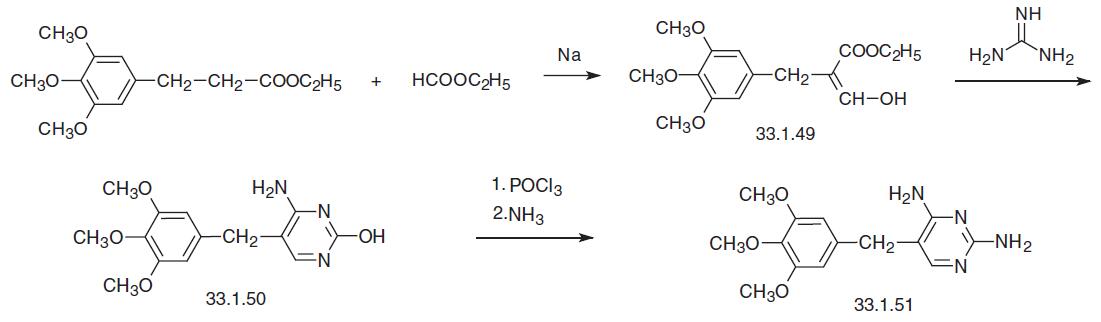
All of the other syntheses begin with 3,4,5-trimethoxybenzaldehyde. According to one of them, condensation of 3,4,5-trimethoxybenzaldehyde with 3-ethoxy- or 3-anilinopropionitrile gives the corresponding benzylidene derivative (33.1.52), which upon direct reaction with guanidine gives trimethoprim.
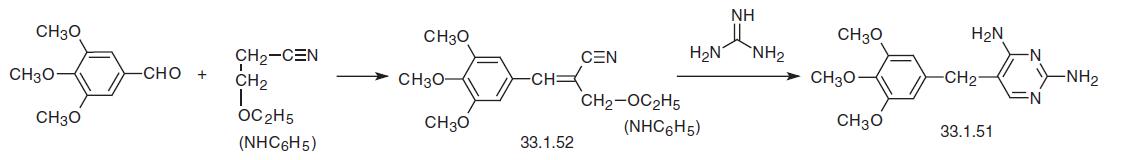
Trimethoprim has also been synthesized by condensing 3,4,5-trimethoxybenzaldehyde with malonic acid dinitrile in a Knoevenagel reaction, which forms the derivative (33.1.53), which is partially reduced to the enamine (33.1.54) by hydrogen using a palladium on carbon catalyst, which upon being reacted with guanidine is transformed into trimethoprim.
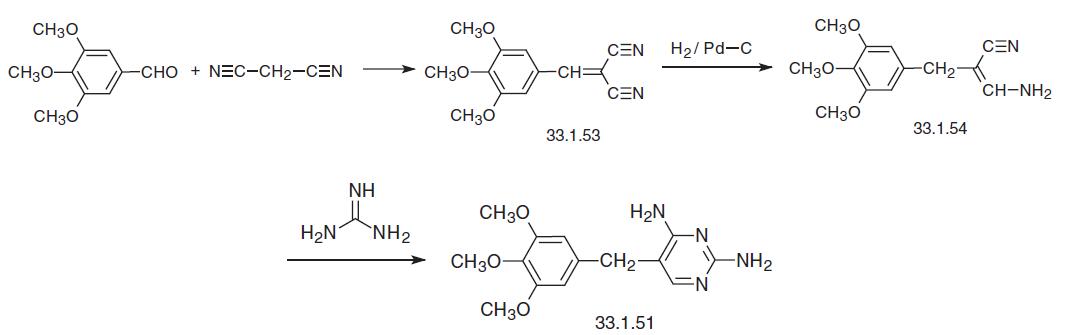
Finally, trimethoprim can be synthesized in a manner that also uses a Knoevenagel condensation of 3,4,5-trimethoxybenzaldehyde as the first step, but this time with ethyl cyano�acetate, which gives an ylidene derivative (33.1.55). The double bond in this product is reduced by hydrogen over a palladium on carbon catalyst, giving 3,4,5-trimethoxybenzylcyanoacetic ester (33.1.56). Reacting this in a heterocyclization reaction with guani�dine gives the desired trimethoprim. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anti-arrhythmics: increased risk of ventricular
arrhythmias with amiodarone - avoid; concentration
of procainamide increased.
Antiepileptics: antifolate effect and concentration of
fosphenytoin and phenytoin increased.
Antimalarials: increased risk of antifolate effect with
pyrimethamine.
Ciclosporin: increased risk of nephrotoxicity;
concentration of ciclosporin reduced by IV
trimethoprim.
Cytotoxics: increased risk of haematological
toxicity with azathioprine, methotrexate and
mercaptopurine; antifolate effect of methotrexate
increased.
Tacrolimus: possible increased risk of nephrotoxicity. | [Metabolism]
About 10 to 20
% of trimethoprim is metabolised in the liver and small amounts are excreted in the faeces via the bile, but most, about 40 to 60
% of a dose, is excreted in urine, mainly as unchanged drug.
Trimethoprim is excreted mainly by the kidneys through glomerular filtration and tubular secretion. | [storage]
Store at 2-8°C |
| Questions And Answer | Back Directory | [Pyrimethamine class antibacterial agents]
Trimethoprim is a lipophilic and weak alkaline pyrimethamine class bacteriostatic agent. It is a white or almost white crystalline powder, odorless, bitter, and slightly soluble in chloroform, ethanol or and acetone, but almost insoluble in water and highly soluble in glacial acetic acid solution. It has an antibacterial spectrum which is similar with sulfa drugs, but with a strong antibacterial effect. It has a good effect on treating Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Staphylococcus saprophyticus, and a variety of other gram-positive and negative bacteria. But it is ineffective against Pseudomonas aeruginosa infection. Its minimum inhibitory concentration is often less than 10 mg/L with using alone being easy to cause bacterial resistance, and thus it is generally not used alone, and mainly combined with sulfa drug to form compound preparation for clinical treatment of urinary tract infections, intestinal infections, respiratory infections, dysentery, enteritis, typhoid fever, meningitis, otitis media, meningitis, sepsis and soft tissue infections. It has an effect on treating typhoid and paratyphoid effect which is not less than ampicillin; It can also be combined with long-acting sulfa drugs for prevention and treatment of drug-resistant falciparum malaria.
The basic principle of anti-bacterial of trimethoprim is to interfere with folate metabolism in bacteria. The main mechanism of action is the selective inhibition of the activity of dihydrofolate reductase in bacteria so that dihydrofolate can’t be reduced to tetrahydrofolate. Since the synthesis of folic acid is the main part of a nucleic acid biosynthesis, and therefore the product prevents bacterial nucleic acids and proteins synthesis. Moreover, the binding affinity of trimethoprim (TMP) to bacterial dihydrofolate reductase enzyme is five times as strong as that to the mammalian dihydrofolate reductase. The combination between it with sulfa drugs can cause dual blockage to the folic acid biosynthesis metabolism of bacteria so that there is a synergistic effect which will enhance the antibacterial activity of sulfa drugs, and can turn antibacterial effect to bactericidal effect which reduce the drug-resistant strains. In addition, the product can also enhance the antibacterial effects of a variety of other antibiotics (such as tetracycline, gentamicin).
| [Side effects]
Trimethoprim (referred to as the TMP) has a low toxicity with commonly used dose causing rare cases of adverse reactions. Since the product can interfere with folate metabolism which may cause patients’ suffer from some adverse reactions of blood systems such as anemia, leukopenia and thrombocytopenia. This is commonly observed in cases of overdose or long duration of application. Therefore, during the treatment, it is necessary to regularly check blood condition. This product has the maximum daily dosage being lower than 0.5g with continuous medication time being less than one week. Upon blood system adverse reaction, the patient can orally administrate folic acid preparation for treatment. This product is not suitable to be simultaneously combined with anticancer drugs, antiepileptic drugs and other folic acid antagonists used; the combination between TMP and SMZ or SD even can cause crystallization of urine. Other adverse reactions also include mild skin rash and gastrointestinal reactions.
| [Chemical Properties]
White crystalline powder, odorless, bitter taste. Melting point: 199-203 °C. It is insoluble in water, ether, benzene, and slightly soluble in chloroform, methanol, highly soluble in acetic acid.
| [Uses]
1. It can be used as a synergistic antimicrobial drugs; it can also be used for treating bacterial infections and coccidiosis in poultry.
2. It is a novel orally administrated broad-spectrum antibiotics. It has a similar antibacterial spectrum with sulfa drugs but with a stronger potency. It is effective in treating a variety of Gram positive and negative bacteria. Since the bacteria is easy to evolve drug resistance to this product, it is not suitable to be used alone as an antimicrobial drug. The combination between trimethoprim and sulfa drugs can enhance the antibacterial activity by several times to several dozens of times. The product is mainly used for being as the synergistic drugs for sulfonamide drugs with a general ratio of 1: 5 for usage. It can also be used as a veterinary drug for treatment of avian sepsis caused by Escherichia coli, salmonellosis, fowl typhoid, cholera, and respiratory system secondary bacterial infections. It can also be used for the treatment of coccidiosis.
3. Application: used as antibacterial synergistic drugs; treat respiratory tract infections, urinary tract infections and intestinal infections when being used alone.
The above information is edited by the chemicalbook of Dai xiongfeng.
| [Production methods]
Use Trimethoxybenzaldehyde as raw material; first condense with methoxypropionitrile to produce 3'4'5'-trimethoxy-2-cyano-3-methoxy-propene; and cyclized together with guanidine nitrate in the presence of methanol/sodium methoxide.
|
|
|