| Identification | More | [Name]
L-Threonine | [CAS]
72-19-5 | [Synonyms]
2-AMINO-3-HYDROXYBUTANOIC ACID
2-AMINO-3-HYDROXYBUTYRIC ACID
(2S,3R)-(-)-2-AMINO-3-HYDROXYBUTYRATE
(2S,3R)-2-AMINO-3-HYDROXYBUTYRIC ACID
(2S,3R)-(-)-THREONINE
H-L-THR-OH
H-THR-OH
H-THR-OH-THREONINE
L-2-AMINO-3-HYDROXYBUTANOIC ACID
L-2-AMINO-3-HYDROXYBUTYRIC ACID
L-A-AMINO-B-HYDROXYBUTYRIC ACID
L-THR
L(-)-THREONINE
L-THREONINE
RARECHEM AB PP 1459
THR
THREONINE
THREONINE, L-
(s)-threonine
Butanoic acid, 2-amino-3-hydroxy-, [R-(R*,S*)]- | [EINECS(EC#)]
200-774-1 | [Molecular Formula]
C4H9NO3 | [MDL Number]
MFCD00064270 | [Molecular Weight]
119.12 | [MOL File]
72-19-5.mol |
| Chemical Properties | Back Directory | [Appearance]
White crystalline powder | [Melting point ]
256 °C (dec.) (lit.) | [alpha ]
-28.4 º (c=6, H2O) | [Boiling point ]
222.38°C (rough estimate) | [density ]
1.3126 (rough estimate) | [FEMA ]
4710 | L-THREONINE | [refractive index ]
-28 ° (C=6, H2O) | [storage temp. ]
Store at RT. | [solubility ]
H2O: 50 mg/mL
| [form ]
powder
| [pka]
2.09(at 25℃) | [color ]
Yellow | [Odor]
ADM L-Threonine is a high quality product specifically designed for the feed industry. Produced from advanced technology, ADM L-Threonine is composed of 100% isomerically pure L-Threonine, which translates into 100% bioavailability for swine, poultry, and other animals.mild savory | [PH]
5-6 (100g/l, H2O, 20℃) | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [Odor Type]
savory | [optical activity]
[α]20/D 28.5±0.5°, c = 5% in H2O | [Water Solubility ]
90 g/L (20 ºC) | [JECFA Number]
2119 | [Merck ]
14,9380 | [BRN ]
1721646 | [InChIKey]
AYFVYJQAPQTCCC-GBXIJSLDSA-N | [LogP]
-2.94 | [CAS DataBase Reference]
72-19-5(CAS DataBase Reference) | [NIST Chemistry Reference]
Threonine(72-19-5) | [EPA Substance Registry System]
72-19-5(EPA Substance) |
| Questions And Answer | Back Directory | [Essential amino acids]
Isolation and identification of Threonine from the fiber protein hydrolyzate by W. C. Rose in 1935, has proved that it was the last essential amino acids. It is extremely important physiological role in animals, because it is the second or third limiting amino acids for livestock. Such as promoting growth, enhance immune function, etc. Balancing the dietary amino acids to make the ratio of close to the ideal protein, and reducing the protein content of livestock feed requirements. Lacking of threonine, may lead to reduce feed intake, growth retardation, decrease feed efficiency, and suppress immune function and other symptoms. In recent years, lysine, methionine synthesis product has widely used in feed, threonine become the limiting factor affected animal performance gradually. Further study of threonine could help to the livestock and poultry production effectively.
Threonine cannot synthesize by animals, however, it is an essential amino acids for them to balance the composition of amino acids precisely to meet the need to animal growth, improve weight and lean meat, reduce the feed conversion. Threonine also can increase the value of feed raw materials of lower amino acid digestibility, and improve the production performance of low-energy feed. Besides, Threonine can reduce feed crude protein levels and improve feed nitrogen utilization, and reduce feed costs. So Threonine can be used for pigs, chickens, ducks and senior aquatic breeding and farming.
L-threonine is based on bio-engineering principles by using corn starch and other raw materials through submerged fermentation, refined and produced feed additives. L-threonine could adjust the balance of amino acid in feed, promote growth, improve meat quality and improve ncrease the value of feed raw materials of lower amino acid digestibility and produce the low protein feed, save protein resources, reduce the cost of feed ingredients, reduce the nitrogen content of manure and urine and decrease the concentration and release rate of animal building ammonia. L-threonine has used to add in piglet feed, pig feed, chicken feed, shrimp feed and eel feed widely.
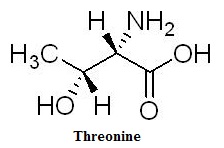
Fig. 1 Threonine Structure | [Physiological function]
Protein is composed of peptides, peptide chain of peptides is composed of amino acids, and amino acids are the constituent units of the protein. Different proteins are made of amino acids in a different order and length of the peptide chain. Actually gene which related to hereditary is an amino acid chain in a different order.
At present, there are 28 kinds of human-related amino acids in total, in these amino acids, there are 9 kinds of amino acids (about 32%) is required from dietary directly, which the human body cannot intake the proteins synthetic by other amino acids. They are leucine, isoleucine, valine, lysine, methionine, tryptophan, threonine, phenylalanine and histidine, while histidine still not clearly. Generally considered that histidine is essential amino acids for the age of 4 infants, while it could be synthesized for elders. Non-essential amino acids are important as well; sometimes their functions are much more important than essential amino acids. Such as taurine, arginine and glutamine, in some disease or stress conditions, they are very important, so called conditionally essential amino acids.
Threonine is essential amino acids, which helps the body maintain protein balance. It plays a role in the formation of collagen and elastin proteins. When threonine, aspartate and methionine combined, they could resist fatty liver. Threonine present in the heart, the central nervous system and skeletal muscle that could prevent the accumulation of fat in the liver. It can promote the production of antibodies to enhance the immune system as well.
In foods, cereals threonine content is low, therefore vegetarian prone to threonine deficiency.
The information received by the Xiaonan editor of Chemicalbook.
| [Metabolism]
Threonine is the only one that not through deamination and transamination in the body's metabolism, but directly catalysed by threonine dehydratase, threonine dehydrogenase and threonine aldolase to other amino acids, such as threonine can be converted to butyryl coenzyme A, succinyl-coenzyme a, serine, glycine, etc. In addition, excessive of threonine can increase activity of lysine-α-keto gluconate reductase, adding the appropriate amount of threonine can be eliminated weight decreased, liver, muscle tissue protein/deoxyribonucleic acid (DNA), ribonucleic acid (RNA)/DNA ratio decreased caused by excessive lysine in the diet. Adding threonine also could reduce the suppression growth caused by of excessive tryptophan, or methionine. According to reports, the chicken to absorb threonine mostly in the duodenum, threonine turned to be liver protein after absorbed in crop and glandular stomach and deposition in the body. However, the detailed mechanisms of how threonine to participate synthesis protein is still unclear.
| [Quantitative analysis]
Weighed sample about 200mg accurately, dissolved in 3ml glacial and 5ml acetic acid, add 2 drops of crystal violet solution (TS-74), with 0.1mol/L perchloric acid titration to green or blue completely disappeared. 1 ml, 0.1mol/L perchloric acid equivalent L-threonine (C4H9NO3) 11.91mg.
| [Toxicity]
LD503098 mg/kg (rats, i.p.).
| [Limited use]
5.0% of total protein mass in food (FDA, §172.320,2000).
| [Chemical Properties]
White with thorhombic or crystalline powder. Odorless, taste sweet
| [Uses]
1.Threonine is an important nutritional supplement which could fortify cereals, pastries, dairy products, restore the body as fatigue, and promote growth and development like tryptophan. Due to its structure contained threonine hydroxyl,which could held the water on human skin and combined with the oligosaccharide chains to protect cell membranes. Threonine plays an important role in the body, promoted phospholipid synthesis and fatty acid oxidation in the Medicine field as well.
2.For biochemical research, the pharmaceutical amino acids could be a nutritional medicine to cure anemia primarily.
3.Amino acid as medicines is mainly used in amino acid infusion, comprehensive amino acid preparations and food nutrition fortifier. Such as the lack of threonine can cause loss of appetite, weight loss, fatty liver, testicular atrophy, anterior pituitary cells, dyeing changes and bone development. Adverse reactions Contraindications: When an adult infusion 22.5g, can cause fever, headaches and other adverse reactions.
4.Nutritional supplements. To meet the need of cereal protein, L-lysine is the first, another is L-threonine. Even though the content of L-threonine is high, the binding between peptide and threonine is difficult to hydrolysis, and not easy to absorb and digest.
It is also could be used in preparation of amino acid infusion and comprehensive amino acid preparations.
5.One of the essential amino acids in human body, it can be used to improve nutrition and physical fitness.
| [Production method]
1.Hydrolysized the natural protein (sericin, casein, fibroin, etc.) with high content of Threonine, separated and refined by ion exchange resin.
DL-threonine were made from glycine copper and acetaldehyde reaction (see "DL-threonine"), in the resulting solution of DL-threonine inoculated within L-type crystals that only carry out the L-optical resolution and get L-histidine.
2.Direct fermentation method
Glucose as raw material, breeding auxotrophic synthesis and structural analogues feedback inhibition and repression to L-threonine acid production was 18g/L, Corynebacterium glutamic acid production was 14g/L, clayey race bacilli rate 14g/L.
Glucose [Brevibacterium, Corynebacterium glutamicum, clayey race bacillus] → L-threonine
3.Chemical synthesis
Threonine is a mixture of four optical isomers after chemical synthesis, named DL-threonine.
L-shaped threonine is the composition of protein, therefore Su should be separated from others, and further isolate the optical isomers to get L-threonine.
similar aldol reaction when Copper with glycine and acetaldehyde under alkaline conditions to get the mixture of Su copper body and other body. According to their stability and solubility, he copper removal can be seprate to DL-threonine, L-threonine was finally isolated.
Synthesis and amplification of the production process are described below.
| [Synthesis process]
Preparation glycine:monochloroacetic acid 189g (2 mol), formaldehyde solution 2100 ml (3.3mol), mixed and cooled to below 10 ℃, concentrated aqueous ammonia was added 750ml (10mol), control dropping, temperature did not exceed 10 ℃. After the addition of ammonia, keep 30 ℃, 4h. Concentrating under pressure to 300-400ml until crystallized. After washing, drying crude glycine, glycine crude were added about 1.5-2 times the amount of water, heated to soluble, add 1% activated charcoal decolorized; filtration, adding 2-2.5 times of the volume of methanol, placed in refrigerator overnight, crystals were collected by filtration and get glycine boutique. The recovery is about 60%-68%.
Preparation of glycine copper
Glycine 100g, added 7L water, heating 60 ℃ to make it soluble. Copper carbonate was slowly added to the solution 80g, at 60 ℃ incubated 1h. Filtrated the solution to remove unreacted copper carbonate before the solution become cold. The filtrate was collected to keep cooling naturally, precipitated blue needle-like crystals (with a molecular crystal water), crystals were collected by filtration and washed, 60 ℃ dried to get glycine copper, recovery is about 95%-98%.
Preparation of threonine copper
Glycine copper 52.5g, added 425ml of methanol and stirred to dissolve, then added 80ml of ethanol at a tempreture below 10 ℃, 5g of sodium hydroxide was added in in 90ml of methanol solution for pre-dissolved until the temperature is no longer raise, and kept it in 60 ℃ for reaction 1h. insolubles were filtered before the solution become cold, the filtrate was added glacial acetic acid 5.5ml, recovering methanol to dryness under reduced pressure, combined with 75ml of methanol was stirred overnight. Crystals were collected by filtration, washed, and dried to get the Su and other bodies threonine mixture of copper, recovery is about 68%-74%.
Copper removal
Threonine copper 427g, added 10% ammonium hydroxide solution 6L, completely dissolved, collected the filtration, adsorption on a cation exchange resin 732, washed with 2mol/L and water until the eluent ninhydrin did not react. Combined eluent and film concentrated to 1.5L, added 3L ethanol to make crystals and placed in the refrigerator overnight, the crystals were collected by filtration to get DL-threonine crude. Recovery is about 62%-73.8%.
DL-threonine crude 42g, added 126ml water to make it soluble by heating, bleached filter the filtrate, added 252ml of ethanol, cooled overnight, filtered and purified crystals were DL-threonine. Recovery is about 87%-91.3%.
Split, refining
the refining of DL-threonine 810g, DL-threonine 90g, added water 2.88L, slowly stirred (approximately 50r/min), and heated to above 95 ℃ until it has completely dissolved. Then cooled to 40 ℃, and 10% D-threonine of total amount of DL-threonine, and slowly cooled to 30 ℃ (15min per drop 1 ℃), crystallize D-threonine. The crystals were collected by filtration, and with 80 ℃ dried to get D-threonine. The filtrate was collected and split into D-threonine out the same amount of DL-threonine (approximately 150-170g), to keep the total volume constant, the same operation with the split D-threonine (but lower temperature is around 40 ℃, added L-threonine) to get L-threonine. This repeated operation can seprate D-threonine and L-threonine.
Merged D-threonine crude and L-threonine crude to refined, namely recrystallization. D-type and L-threonine addedwith water 4 times respectively, the solution was heated to 90 ℃, 1% active carbon, filtrate was collected before it cooled, added 2 times of volume of ethanol to cool, stirring occasionally, to precipitate crystals get D-threonine and L-threonine boutique, recovery is around 87.3%-91.6%.
L-threonine content of more than 95%, [α] 20D-26 °--29 °, paper chromatography showed one spot.
| [Amplification of the production process]
Glycine as the raw material, reacted with basic copper carbonate produced to be copper glycine firstly when α-H was active, glycine copper with acetaldehyde under alkaline conditions could coour similar effects cross aldol condensation to produce DL-threonine copper (including Su body and red body), and by ion exchange, isolated copper removal from Su body DL-threonine, and finally induced crystallization method by using D-threonine and L--threonine Split . Its reaction is as follows:
Preparation of glycine copper in a 500L reactor, added 50kg glycine, 350L water and 40kg basic copper sulfate incubated 60 ℃ for 1h, filtered off the unreacted copper salt, collected the filtrate and cooled overnight, crystals were collected by filtration, dried in 60 ℃ to obtain blue glycine copper.
Preparation of threonine copper in the 1000L reactior, added 75kg of copper glycinate and 600L methanol, stirred to dissolve, added 120L acetaldehyde, 90L 5% KOH methanol solution, stirred 1h in 60 ℃, the insoluble matter was collected by filtration. added 5.5L glacial acetic acid into filtrate. Dried methanol to a recovery vacuum, added 75L water and keep in 5 ℃ overnight, crystals were collected by filtration, washed, drained the mixed threonine copper.
Concentrated, refined
Added DL-threonine crude 40kg into the 500L reactor, and DL-threonine as raw materials in alkaline conditions to react with chlorine chloride, extracted with acetone and concentrated under reduced pressure, and finally by the acylase I split the products.
|
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [RTECS ]
XO8590000
| [Hazard Note ]
Irritant | [TSCA ]
Yes | [HS Code ]
29225000 | [Safety Profile]
Moderately toxic by
intraperitoneal route. When heated to
decomposition it emits toxic fumes of NOx. | [Hazardous Substances Data]
72-19-5(Hazardous Substances Data) |
| Hazard Information | Back Directory | [Definition]
ChEBI: An optically active form of threonine having L-configuration. | [Brand name]
L -Threonine is JAN. | [Biotechnological Production]
L-Threonine can be produced using strains of E. coli or C. glutamicum. As threonine
is also an amino acid of the aspartate family, aspartate semialdehyde is a common
intermediate with the biosynthesis of L-lysine. In order to optimize a high-yielding
L-threonine–producing strain, the following strategy is applied: the pathway
towards L-lysine is minimized by reducing the activity of dihydrodipicolinate
synthase (dapA) and at the same time the pathway towards L-threonine is favored by
overexpression of the genes of the threonine operon, which consists of the genes for
homoserine dehydratase (thrA), homoserine kinase (thrB), and threonine synthase
(thrC). As L-threonine is also a precursor for L-isoleucine, further conversion of
L-threonine into L-isoleucine has to be minimized by deactivation of the threonine
dehydratase gene (ilvA). In the meantime, E. coli based strains have also been
developed by the application of systems biology, not only by deletion or
downregulation of the competing pathways such as L-lysine, L-methionine, and
L-isoleucine, but also by optimization of the supply of key precursors such as
oxaloacetate. The E. coli strain has been reported to produce 82 g/L L-threonine in
48 h with a carbon yield of 39 %. A more detailed description of the development
of a commercial L-threonine process has been given by Debabov.
Today L-threonine is manufactured on a commercial scale of several thousand
tonnes using the E. coli fermentation process. | [General Description]
This certified reference material (CRM) is produced and certified in accordance with ISO/IEC 17025 and ISO 17034. This CRM is traceable to primary material from an NMI, e.g. NIST or NMIJ.
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com. | [Biochem/physiol Actions]
L-Threonine is considered to be an essential amino acid. L-Threonine is involved in the synthesis of mucin, a protein supporting intestinal function and integrity. L-Threonine is required for the performance of the immune system. It is also essential for O-linked glycosylation, protein phosphorylation and glycine synthesis. | [Purification Methods]
Likely impurities are allo-threonine and glycine. Crystallise L-threonine from H2O by adding 4volumes of EtOH. Dry and store it in a desiccator. It also crystallises from 80% EtOH to give hexagonal plates m 262-263o(dec). It sublimes at 200-226o/0.3mm with 99.6% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. [Elliot J Chem Soc 62 1950, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 1 pp 176-183, Vol 3 pp 2238-2257 1961, Beilstein 4 IV 3171.] |
|
|