| Identification | Back Directory | [Name]
Boc-Glycine hydrazide | [CAS]
6926-09-6 | [Synonyms]
BUTTPARK 77\04-08
BOC-GLYCINE HYDRAZIDE
Boc-Glycinehydrazide8%
Boc-Glycinehydrazide���,98%
Butoxycarbonylglycine hydrazide
Boc-Glycine hydrazide USP/EP/BP
N-TERT-BUTOXYCARBONYLGLYCYLHYDRAZIDE
tert-Butyl (2-hydrazinyl-2-oxoethyl)
2-Aminoacetohydrazide, 2-BOC protected
tert-butyl (2-hydrazino-2-oxoethyl)carbamate
ert-butylN-(2-hydrazinyl-2-oxoethyl)carbamate
tert-butyl N-(2-hydrazino-2-oxo-ethyl)carbamate
tert-butyl N-(2-hydrazinyl-2-oxoethyl)carbamate
tert-butyl N-(2-hydrazinyl-2-oxo-ethyl)carbamate
tert-butyl N-[(hydrazinecarbonyl)methyl]carbamate
Glycine, N-[(1,1-dimethylethoxy)carbonyl]-, hydrazide
Hydrazinocarbonylmethyl-carbamic acid tert-butyl ester
N-(2-hydrazino-2-oxoethyl)carbamic acid tert-butyl ester
N-(2-hydrazino-2-keto-ethyl)carbamic acid tert-butyl ester
HydrazinocarbonylMethyl-carbaMic acid tert-butyl ester, 95%+
tert-Butyl (2-hydrazino-2-oxoethyl)carbamate, 2-[(tert-Butoxycarbonyl)amino]acetohydrazide | [Molecular Formula]
C7H15N3O3 | [MDL Number]
MFCD01570558 | [MOL File]
6926-09-6.mol | [Molecular Weight]
189.21 |
| Chemical Properties | Back Directory | [Melting point ]
111-115 | [Boiling point ]
386.5±25.0 °C(Predicted) | [density ]
1.139±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [pka]
11.40±0.46(Predicted) |
| Hazard Information | Back Directory | [Uses]
Boc-Glycine hydrazide is used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | [Synthesis]
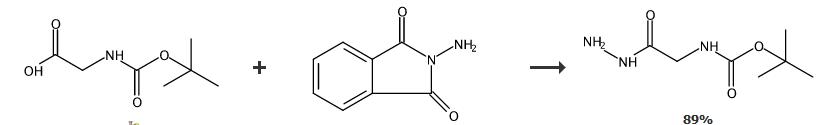
N-Protected Hydrazides 2a-l; General Procedure The protected amino acid 1 (2 equiv) and EDAC (2 equiv) were dissolved in the minimum amount of CH2Cl2 at 0 ??C. The mixture was stirred at 0 ??C for 2 h. PhthNNH2 (1 equiv) was added and the mixture was stirred at 0 ??C for 1 h and then at r.t. overnight. Distillation of the solvent under reduced pressure gave a product which was dissolved in EtOAc and washed successively with 1 M KHSO4, sat. aq NaHCO3, and brine. The organic layer was then dried (Na2SO4), filtered, and concentrated in vacuo. The residue was precipitated with Et2O to yield compound 2. If necessary, the residue was purified by column chromatography (silica gel, hexane- EtOAc, 3:7 to 7:3). Compounds 4a-l; General Procedure Aminomethylated polystyrene resin (3 equiv; 1.1 mmol/g, 100-200 mesh) was conditioned for 10 min at r.t. in CH2Cl2. Then 2 (1 equiv) was added, and the mixture was slowly stirred for 24 h at r.t. The mixture was filtered and the solvent was evaporated in vacuo to give product 4. Compound 4h, yield 89%. |
|
|