| Identification | More | [Name]
Diphenolic acid | [CAS]
126-00-1 | [Synonyms]
4,4-BIS(4-HYDROXYPHENYL)-N-VALERIC ACID
4,4-BIS(4-HYDROXYPHENYL)PENTANOIC ACID
4,4-BIS(4-HYDROXYPHENYL)VALERIC ACID
4,4-BIS(P-HYDROXYPHENYL)PENTANOIC ACID
4,4'-BIS(P-HYDROXYPHENYL)VALERIC ACID
4,4-BIS(P-HYDROXYPHENYL)VALERIC ACID
CTFA
DIPHENOLIC ACID
G,G-BIS(4-HYDROXYPHENYL)VALERIC ACID
TIMTEC-BB SBB009924
,-Bis(p-hydroxyphenyl)valericacid
4,
4,4-bis(4-hydroxyphenyl)valeric
4-Hydroxy-(4-hydroxyphenyl)benzenebutanoicacid
4-hydroxy-gamma-(4-hydroxyphenyl)-gamma-methyl-benzenebutanoicaci
4,4-BIS(4-HYDROXYPHENYL)VALERIC ACID, 95 %
4,4-BIS-(4-HYDROXYPHENYL)-N-VALERICACID(DIPHENOLICACID)
4-hydroxy-gamma-(4-hydroxyphenyl)-gamma-methyl-benzenebutanoic acid
4,4-Di(4'-hydroxyphenyl)pentanoic acid
4-Hydroxy-γ-(4-hydroxyphenyl)-γ-methylbenzenebutanoic acid | [EINECS(EC#)]
204-763-2 | [Molecular Formula]
C17H18O4 | [MDL Number]
MFCD00002800 | [Molecular Weight]
286.32 | [MOL File]
126-00-1.mol |
| Chemical Properties | Back Directory | [Melting point ]
167-170 °C(lit.)
| [Boiling point ]
388.69°C (rough estimate) | [density ]
1.30 | [refractive index ]
1.5450 (estimate) | [Fp ]
208°C | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
4.66±0.10(Predicted) | [color ]
White to Off-White | [Merck ]
14,3312 | [BRN ]
1886361 | [InChIKey]
VKOUCJUTMGHNOR-UHFFFAOYSA-N | [LogP]
2.890 (est) | [CAS DataBase Reference]
126-00-1(CAS DataBase Reference) | [EPA Substance Registry System]
126-00-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R36:Irritating to the eyes. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [F ]
10 | [TSCA ]
Yes | [HS Code ]
29182900 |
| Hazard Information | Back Directory | [Chemical Properties]
Light pink solid | [Uses]
Diphenolic acid (DPA) has been identified as a potential replacement for bisphenol A, which is one of the monomers for epoxy resins and polycarbonates. DPA also can Intermediate for surface coatings, lubricating oil additives, cosmetics, surfactants, plasticizers, textile chemicals. | [Definition]
ChEBI: Diphenolic acid is a bisphenol. | [Preparation]
Diphenolic acid (DPA) can be made by the condensation reaction of levulinic acid with phenol in the presence of acid catalysts.
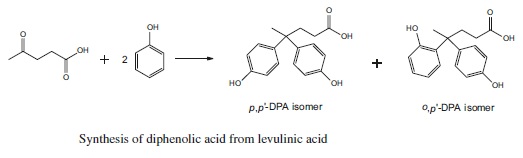
Synthesis of diphenolic acid from levulinic acid
| [Industrial uses]
Diphenolic acid, the condensation product of levulinic acid and phenol, is useful in the preparation of the modified phenol formaldehyde resins, polyether resins and as monocarboxilic acid chain stopper in alkyd resin (Bader, 1960). Further, diphenolic acid can be substituted for bis-phenol A, the primary raw material for production of epoxy resin. | [Purification Methods]
When recrystallised from *C6H6, the crystals have 0.5 mol of *C6H6 (m 120-122o), and when recrystallised from toluene, the crystals have 0.5 mol of toluene. Purify the acid by recrystallisation from hot H2O. It is soluble in Me2CO, AcOH, EtOH, propan-2-ol, methyl ethyl ketone. It can also be recrystallised from AcOH, heptane/Et2O or Me2CO/*C6H6. It has max 225 and 279nm in EtOH. The methyl ester has m 87-89o (aqueous MeOH to give the trihydrate). [Bader & Kantowicz J Am Chem Soc 76 4465 1954, Beilstein 10 IV 1890.] |
|
|