| Identification | More | [Name]
3-Buten-1-ol | [CAS]
627-27-0 | [Synonyms]
1-HYDROXY-3-BUTENE
3-BUTEN-1-OL
3-BUTENE-1-OL
4-HYDROXY-1-BUTENE
ALLYLCARBINOL
1-Buten-4-ol
3-buten-1-0l
3-Buten-1-O1
3-butene-1-0l
3-Butenol
3-Butenyl alcohol
BUTEN-(3)-O1-(L)
CH2=CHCH2CH2OH
Vinylethyl alcohol
3-BUTEN-1-OL (1-4)
3-BUTEN-1-OL 96+%
3-BUTEN-1-OL 98+%
3-BUTEN-1-OL 99+%
b-Vinylethanol
2-Vinylethanol | [EINECS(EC#)]
210-991-3 | [Molecular Formula]
C4H8O | [MDL Number]
MFCD00002959 | [Molecular Weight]
72.11 | [MOL File]
627-27-0.mol |
| Chemical Properties | Back Directory | [Appearance]
liquid | [Melting point ]
-31.44°C (estimate) | [Boiling point ]
112-114 °C (lit.) | [density ]
0.838 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.421(lit.)
| [Fp ]
90 °F
| [storage temp. ]
Flammables area | [solubility ]
soluble in Chloroform, Methanol | [form ]
Liquid | [pka]
15.04±0.10(Predicted) | [color ]
Clear colorless to slightly yellow | [Specific Gravity]
0.843 | [Stability:]
Stable. Incompatible with acids, acid chlorides, acid anhydrides, oxidizing agents. Flammable. | [explosive limit]
2-28%(V) | [Water Solubility ]
SOLUBLE | [BRN ]
1633504 | [InChIKey]
ZSPTYLOMNJNZNG-UHFFFAOYSA-N | [LogP]
0.679 (est) | [CAS DataBase Reference]
627-27-0(CAS DataBase Reference) | [NIST Chemistry Reference]
3-Buten-1-ol(627-27-0) | [EPA Substance Registry System]
627-27-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R10:Flammable.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
UN 1987 3/PG 3
| [WGK Germany ]
3
| [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
II | [HS Code ]
29052990 |
| Questions And Answer | Back Directory | [Description]
3-Buten-1-ol(627-27-0) is a homoallyl alcohol. It is employed in a study of the Mn-catalyzed hydrohydrazination of olefins. It is also used in a study of the conversion of propargylic acetates to ethers catalyzed by ferric chloride1. It can also be used as a starting reagent in asymmetric total synthesis of natural seimatopolide B. It was also used in the synthesis of catalytic bimetallic nanoparticles2.
|
| Hazard Information | Back Directory | [Chemical Properties]
liquid | [Uses]
3-Buten-1-ol(627-27-0) is an aliphatic primary alcohol used as a reagent in organic synthesis.
| [Definition]
ChEBI: 3-Buten-1-ol is a primary alcohol. | [General Description]
3-Buten-1-ol(627-27-0) is a homoallyl alcohol that can be prepared by the dehydration of 1,4-butanediol using cerium catalyst. The intramolecular hydrogen bonding of 3-buten-1-ol has been studied using FT-IR and 1H NMR spectroscopic data. Its microwave spectrum has been recorded and analyzed. The alkylation reaction of 3-buten-1-ol using titanium-organoaluminum system has been studied. Its gas-phase enthalpy of formation has been reported to be -147.3 ± 1.8kJ mol-1.
| [Hazard]
3-Buten-1-ol is strongly irritating to the skin and eyes. it may cause respiratory irritation. it is toxic to aquatic organisms and has long lasting effects. | [Synthesis]
The synthesis of 3-Buten-1-ol is as follows:After adding 24.3 g (1 mol) of magnesium turnings, 50 g of diethyl ether and 1 ml of dibromoethane in a 500 mL four-necked flask,A solution of 62.5 g of vinyl chloride (1 mol) dissolved in 200 g of diethyl ether was added dropwise to magnesium and diethyl ether, and the micro reflux was controlled, and the dropwise addition was completed, and the mixture was kept under a micro reflux for 2 hours, and the temperature was lowered to -5 °C;Then 44 g (1 mol) of ethylene oxide was dissolved in 60 g of diethyl ether. Ethylene oxide is added dropwise to the vinylmagnesium chloride solution prepared above, control the temperature -5-5 degrees, and stir at this temperature for 1 hour. Sampling gas phase analysis until the reaction is complete; pour the reaction solution into a 1000 mL beaker, Add 200mL of ice water and add ammonium chloride until the residual magnesium disappears. Control the temperature 0-10 ° C, stir for 1 hour after the addition, add salt to saturation, static layering. The organic layer was separated and washed twice with 100 ml of saturated brine, and a polymerization inhibitor was added. The product is subjected to rectification to obtain 53 g of 3-buten-1-ol, and the content is 99.2%. The yield was 73.6%.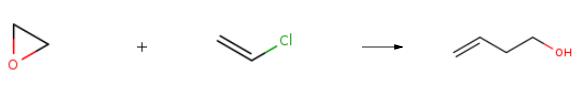
|
|
|