| Identification | More | [Name]
Trisodium citrate dihydrate | [CAS]
6132-04-3 | [Synonyms]
2-HYDROXY-1,2,3-PROPANETRICARBOXYLIC ACID, MONOHYDRATE
2-HYDROXY-1,2,3-PROPANETRICARBOXYLIC ACID, TRISODIUM SALT, DIHYDRATE
2-hydroxytricarballylic acid
ACIDUM CITRICUM MONOHYDRICUM
ANTI-BABOON IGM, BIOTIN
BETA-HYDROXY-TRICARBOXYLIC ACID MONOHYDRATE
CITRIC ACID
CITRIC ACID-1-HYDRATE
CITRIC ACID, 3NA, DIHYDRATE
citric acid anhydrous
CITRIC ACID H2O
CITRIC ACID MONOHYDRATE
CITRIC ACID NA3-SALT 2H2O
CITRIC ACID TRISODIUM SALT DIHYDRATE
HYDROXYTRICARBALLYLIC ACID MONOHYDRATE
NATRII CITRAS
SODIUM CITRADE DIHYDRATE
SODIUM CITRATE
SODIUM CITRATE 2H2O
SODIUM CITRATE, DIHYDRATE | [EINECS(EC#)]
201-069-1 | [Molecular Formula]
C6H9Na3O9 | [MDL Number]
MFCD00150031 | [Molecular Weight]
294.1 | [MOL File]
6132-04-3.mol |
| Chemical Properties | Back Directory | [Appearance]
white powder or colourless crystals | [Melting point ]
>300 °C(lit.)
| [bulk density]
600kg/m3 | [density ]
1.76
| [FEMA ]
3026 | [Fp ]
173.9 °C
| [storage temp. ]
Store at RT. | [solubility ]
H2O: 100 mg/mL
| [form ]
powder
| [pka]
6.40 | [color ]
white
| [Odor]
Odorless | [PH]
7.0-9.0 (25℃, 50mg/mL in H2O) | [PH Range]
7.5 - 9 at 29.4 g/l at 25 °C | [Stability:]
Stable. Incompatible with bases, reducing agents, oxidizing agents. | [biological source]
synthetic | [Water Solubility ]
720 g/L (25 ºC) | [λmax]
λ: 260 nm Amax: 0.01
λ: 280 nm Amax: 0.01 | [Merck ]
14,8602 | [BRN ]
6104939 | [InChIKey]
NLJMYIDDQXHKNR-UHFFFAOYSA-K | [LogP]
-1.72 | [CAS DataBase Reference]
6132-04-3(CAS DataBase Reference) | [EPA Substance Registry System]
6132-04-3(EPA Substance) | [Absorption]
≤0.015 at 260nm
≤0.015 at 280nm |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R37/38:Irritating to respiratory system and skin .
R41:Risk of serious damage to eyes.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing . | [WGK Germany ]
1
| [RTECS ]
GE7810000
| [TSCA ]
Yes | [HS Code ]
29181500 | [Toxicity]
LD50 orally in Rabbit: > 8000 mg/kg |
| Questions And Answer | Back Directory | [General description]
Sodium citrate, (molecular formula: Na3C6H5O7 • 2H2O) has molecular weight of 294.1, is a colorless crystal or white crystalline powder product; it is odorless, salty taste, and cool.It will lose its crystal water at 150 °C and will be decomposed at even higher temperature. It also has slight deliquescence in wet air and has weathering property upon hot air. It is soluble in water and glycerol, but insoluble in alcohol and some other organic solvents. Sodium citrate has no toxic effect, and has pH adjusting capability as well as having a good stability, and therefore can be used in the food industry. Sodium citrate has the greatest demand when being used as a food additive; As food additives, it is mainly used as flavoring agents, buffers, emulsifiers, bulking agents, stabilizers and preservatives; in addition, combination between sodium citrate and citric acid can be used in a variety of jams, jelly, juice, drinks, cold drinks, dairy products and pastries gelling agents, flavoring agents and nutritional supplements.
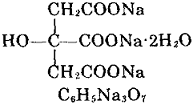 | [Excellent performance]
Sodium citrate is currently the most important citrate. It is produced by two steps: first starch food is fermented to generate citric acid; secondly, citric acid is neutralized by alkali to generate the final products. Sodium citrate has the following excellent performance:
- Safe and nontoxic properties; Since the basic raw material for the preparation of sodium citrate mainly comes from the food, it is absolutely safe and reliable without causing harm to human health. The United Nations Food and Agriculture and the World Health Organization has no restriction in its daily intake, which means that this product can be considered as non-toxic food.
- It is biodegradable. After subjecting to the dilution of a large amount of water, sodium citrate is partially converted into citrate, which coexists with sodium citrate in the same system. Citrate is easy to subject to biological degradation at water by the action of oxygen, heat, light, bacteria and microbes. Its decomposition pathways are generally going through aconitic acid, itaconic acid, citraconic acid anhydride to be further converted to carbon dioxide and water.
- The ability of forming complex with metal ions. Sodium citrate has a good capability of forming complex with some metal ions such as Ca2+, Mg2+; for other ions such as Fe2+, it also has a good complex-forming ability.
- Excellent solubility, and the solubility increases with increasing temperature of water.
- It has a good capability for pH adjustment and a good buffering property. Sodium citrate is a weak acid-strong alkali salt; When combined with citrate, they can form a pH buffer with strong compatibility; therefore, this is very useful for some cases in which it is not suitable to have large change of pH value. In addition, sodium citrate also has excellent retardation performance and stability.
The above information is edited by the chemicalbook of Dai xiongfeng.
| [Reference quality standards]
British Pharmacopoeia BP98 Edition
Quality Item Technical Index
Content 99.0-101.0%
PH Meet the rule
Heavy Metal (PB) ≤ 0.001%
Arsenic ≤ 0.0001%
Ferric ≤ 0.001%
Oxalate ≤ 0.03%
Sulfate ≤ 0.015%
Readily carbonizable substance Meet the rule
Chloride ≤ 0.005%
Water 11.0-13.0%
Appearance of the solution clear and transparent solution, colorless
Pyrogen consistent with the test
| [Effect and application]
During the process of clinically taking fresh blood, adding some amount of sterile sodium citrate can play a role in prevent blood clotting; this is exactly taking advantage of the features that calcium citrate can form soluble complexes with calcium ion; In the field of medicine, it is used for the in vitro anti-clotting drugs and anticoagulants drugs, phlegm drugs, and diuretics drugs during blood transfusions; it can also used for cyanide-free electroplating industry; also used as developer for photographic industry. It can be used as flavoring agents, buffering materials, emulsifiers, and stabilizer in the food industry. Moreover, it is also widely used in chemical, metallurgical industry, the absorption of sulfur dioxide exhaust with the absorption rate of 99% and regenerate liquid sulfur dioxide citrate for recycle application. Sodium citrate has a good water solubility and a excellent cheating capability with Ca2 +, Mg2 + and other metal ions; it is biodegradable and has a strong dispersing ability and anti-redeposition ability; Daily-applied chemical detergents use it as alternative to trimer sodium phosphate for production of non-phosphorus detergent and phosphate-free liquid detergent. Adding a certain amount sodium citrate to the detergent can significantly increase the cleaning ability of detergent cleaning. The large scale of application of sodium tripolyphosphate as a builder in detergents is an important discovery in synthetic detergent industry. It is non-toxic without environmental pollution; it can also be acted as a buffer for the production of cosmetics.
 | [Chemical Properties]
It is colorless crystals or white crystalline powder, and is odorless, cool and salty. It has no melting point with a relative density of 1.857. It is stable in air at room temperature with loss of crystal water when being heated to 150 °C loss of crystal water; further heating will cause its decomposition. It is insoluble in ethanol but highly soluble in water. 5% aqueous solution has a pH value of 7.6 to 8.6. Rats by intraperitoneal injection: LD50: 1549 mg/kg; ADI without any special provisions (FAOWHO, 1994).
| [Uses]
- It can be used as Ph adjusting agents and emulsifying enhancers applied to jam, candy, jelly and ice cream; its combination with citric acid has an effect of alleviating tour; it also has effects on forming complex with metal ions. China rules that it can be applied to various types of food with appropriate usage according to the absolute necessity.
- It can be used as a food additive, as complex agent and buffering agent in electroplating industry; at the field of pharmaceutical industry, it is used for the manufacturing of anti-clotting drugs; and used as the detergent additives in light industry.
- It is used as the analysis agents used for chromatography analysis and can also used for preparing bacterial culture medium; moreover, it can also be applied into pharmaceutical industry.
- The product can be used for the flavoring processing of food, as stabilizers, buffers and deputy complex-forming agents in non-toxic electroplating industry; at pharmaceutical industry, it is used as anti-clotting agent, phlegm drugs and diuretics drugs. It can also be used in brewing, injection, newspaper and movies medicines.
 | [Production methods]
It is produced by the neutralization of citric acid by sodium hydroxide or sodium bicarbonate. Dissolve sodium bicarbonate in water upon stirring and heating; add citric acid, continue to heat up to 85-90 °C; adjust the pH to 6.8; adjust active carbon for bleaching. Filter when the mixture is still hot; condense the filtrate under reduced pressure; cool and the crystal comes out; filter, wash, dry to obtain the final products of sodium citrate.
C6H8O7 + 3NaHCO3 → C6H5Na3O7 ? 2H2O + 3CO2 ↑ + H2O
|
| Hazard Information | Back Directory | [Definition]
ChEBI: The dihydrate of trisodium citrate. | [Pharmaceutical Applications]
Sodium citrate, as either the dihydrate or anhydrous material, is
widely used in pharmaceutical formulations.
It is used in food products, primarily to adjust the pH of
solutions. It is also used as a sequestering agent. The anhydrous
material is used in effervescent tablet formulations. Sodium citrate
is additionally used as a blood anticoagulant either alone or in
combination with other citrates such as disodium hydrogen citrate.
Therapeutically, sodium citrate is used to relieve the painful
irritation caused by cystitis, and also to treat dehydration and
acidosis due to diarrhea. | [Biological Activity]
Commonly used laboratory reagent | [Biochem/physiol Actions]
Sodium citrate can act as a buffering agent, resisting changes in pH. Used in blood collection tubes, the citrate chelates calcium ions in blood and thereby disrupts blood clotting. Citrate is a intermediate in the TCA cycle and fatty acid synthesis. Citrate is an allosteric modulator of acetyl-CoA carboxylase, the enzyme that regulates the conversion of acetyl-CoA to malonyl-CoA. | [Safety]
After ingestion, sodium citrate is absorbed and metabolized to
bicarbonate. Although it is generally regarded as a nontoxic and
nonirritant excipient, excessive consumption may cause gastrointestinal
discomfort or diarrhea. Therapeutically, in adults, up to
15 g daily of sodium citrate dihydrate may be administered orally, in
divided doses, as an aqueous solution to relieve the painful irritation
caused by cystitis.
Citrates and citric acid enhance intestinal aluminum absorption
in renal patients, which may lead to increased, harmful serum
aluminum levels. It has therefore been suggested that patients with
renal failure taking aluminum compounds to control phosphate
absorption should not be prescribed citrate- or citric acid-containing
products. | [storage]
Sodium citrate dihydrate is a stable material. Aqueous solutions
may be sterilized by autoclaving. On storage, aqueous solutions may cause the separation of small, solid particles from glass
containers.
The bulk material should be stored in an airtight container in a
cool, dry place. | [Purification Methods]
Crystallise the salt from warm water by cooling to 0o. [Beilstein 3 III 1100, 3 IV 1274.] | [Incompatibilities]
Aqueous solutions are slightly alkaline and will react with acidic
substances. Alkaloidal salts may be precipitated from their aqueous
or hydro-alcohol solutions. Calcium and strontium salts will cause
precipitation of the corresponding citrates. Other incompatibilities
include bases, reducing agents, and oxidizing agents. | [Regulatory Status]
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (inhalations;
injections; ophthalmic products; oral solutions, suspensions, syrups
and tablets; nasal, otic, rectal, topical, transdermal, and vaginal preparations). Included in nonparenteral and parenteral medicines
licensed in the UK. Included in the Canadian List of Acceptable
Non-medicinal Ingredients. |
|
|