| Identification | More | [Name]
Sodium triacetoxyborohydride | [CAS]
56553-60-7 | [Synonyms]
SODIUM TRIACETOXYBOROHYDRIDE
SODIUM TRIACETOXYHYDROBORATE
stab
sodiumtriacetoxyborohydride,calselect?stab
Sodium Triacetoxyborohyride
Sodium triacetoborohydride
Sodium triacetohydroborate, Triacetohydroborate sodium salt
Sodiumtriacetoxyborohydride,95%
Triacetoxyborohydride sodium salt
Sodiumtriacetoxyborohydride,min.95%
Borate(1-), tris(acetato-.kappa.O)hydro-, sodium, (T-4)-
Sodium triacetate boron hydride
Sodium triacetyloxy-hydridoboron
Sodium triacetoxyborohydride ,97%
Sodium triacetoxyborohydride, min. 95%
Sodium triacetoxyborohydride(STAB)
Triacetoxyborohydride sodium salt
"Sodium triacetoxyborohydride(STAB)
Sodium triacetoxy | [EINECS(EC#)]
411-950-4 | [Molecular Formula]
C6H10BNaO6 | [MDL Number]
MFCD00012211 | [Molecular Weight]
211.94 | [MOL File]
56553-60-7.mol |
| Chemical Properties | Back Directory | [Appearance]
white powder | [Melting point ]
116-120 °C (dec.) (lit.) | [Boiling point ]
111.1℃[at 101 325 Pa] | [density ]
1.36[at 20℃] | [vapor pressure ]
0Pa at 25℃ | [storage temp. ]
Flammables + water-Freezer (-20°C)e area | [solubility ]
Soluble in dimethyl sulfoxide, methanol, benzene, toluene, terahydrofuran, dioxane and methylene chloride. | [form ]
Powder | [color ]
White | [Water Solubility ]
reacts | [Sensitive ]
Moisture Sensitive | [Detection Methods]
T�����,NMR,MS | [Merck ]
8695 | [BRN ]
4047608 | [InChIKey]
HHYFEYBWNZJVFQ-UHFFFAOYSA-N | [LogP]
-2.88--1.09 at 20℃ | [CAS DataBase Reference]
56553-60-7(CAS DataBase Reference) | [EPA Substance Registry System]
56553-60-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F,Xi,C | [Risk Statements ]
R15:Contact with water liberates extremely flammable gases.
R34:Causes burns.
R14/15:Reacts violently with water, liberating extremely flammable gases . | [Safety Statements ]
S43:In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add-Never use water) .
S7/8:Keep container tightly closed and dry .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN 1409 4.3/PG 2
| [WGK Germany ]
3
| [F ]
10-21 | [Hazard Note ]
Irritant/Flammable | [TSCA ]
Yes | [HazardClass ]
4.3 | [PackingGroup ]
III | [HS Code ]
29209090 |
| Hazard Information | Back Directory | [Description]
Sodium triacetoxyborohydride(56553-60-7), also known as sodium triacetoxyhydroborate, commonly abbreviated STAB, is a white hygroscopic solid that emits hydrogen gas when hydrolysed in water, which is flammable. STAB is a milder reducing agent than sodium borohydride or even sodium cyanoborohydride. Even sodium cyanoborohydride is a milder reducing agent than sodium borohydride, and is particularly suitable for the reductive amination of aldehydes and ketones. It can reduce aldehydes but not most ketones. It can also be used in the reductive alkylation of secondary amines in aldehyde-bisulfite adducts.
| [Chemical Properties]
white powder | [Uses]
Sodium Triacetoxyborohydride(STAB,56553-60-7) is a hydride reagent used in stereoselective reductive amination. It is able to replace toxic sodium cyanoborohydride under most conditions. It is selective in reducing aldehydes to alcohols in the presence of ketones. STAB is also stable in anhydrous acids, which enables reductive amination of aldehydes and ketones. It used in reductive amination of ketones and aldehydes and reductive amination/lactamization of carbonyl compounds with amines. The advantage of STAB compared to sodium cyanoborohydride is evident. STAB, being non-toxic, is easier to handle and forms no toxic by-products, making the treatment of process waste after the reaction simple and less costly.
| [Application]
Sodium triacetoxyborohydride(56553-60-7) is a mild reagent that exhibits remarkable selectivity as a reducing agent. It reduces aldehydes but not ketones; however, beta-hydroxyketones can be reduced selectively to give 1,3-trans diols.The steric and the electron-withdrawing effects of the three acetoxy groups stabilize the boron-hydrogen bond and are responsible for its mild reducing properties.
Sodium triacetoxyborohydride or [NaBH(OAc)3] can be used as a reagent:
In the reductive amination of ketones and aldehydes.
To prepare N-benzyl-γ-valerolactam by reacting with methyl 4-oxopentanoate and benzylamine via reductive amination/lactamization.
To reduce imines and enamines to corresponding amines.
To reduce quinolines and isoquinolines to corresponding tetrahydro derivatives.
In the hydroboration of alkenes.
To synthesize nitroxide biradicals for creating high relaxivity terminal groups linkage to dendrimers.
| [Reactions]
Sodium Triacetoxyborohydride(56553-60-7) is selective reducing agent in organic synthesis. It is especially suitable for reductive aminations. Since the reaction rate for the reduction of iminium ions is much faster than for ketones or even aldehydes, the reductive amination can be carried out as a one-pot procedure by introducing the reducing agent into a mixture of the amine and carbonyl compound. The presence of a stoichiometric amount of acetic acid, which catalyzes the imine formation and provides the iminium ion, doesn't present any problem under these conditions.
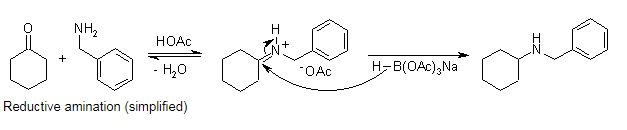
Reductive amination (simplified)
|
|
|