| Identification | More | [Name]
2-Amino-1,3-propanediol | [CAS]
534-03-2 | [Synonyms]
2-AMINO-1,3-PROPANDIOL
2-AMINO-1,3-PROPANEDIOL
2-AMINOPROPANE-1,3-DIOL
DL-SERINOL
SERINOL
1,3-Propanediol, 2-amino-
2-Aminopropan-1,3-propanediol
2-Amino-1,3-dihydroxypropane
Serinol[2-Amino-1,3-Propandiol]
S8002Dl-Serinol
2-Amino-1,3-Propanediol(Serinol)
2-Amino-1,3-propanediol 99%
2-Aminopropan-1,3-diol
2-AMINO-1,3-PROPANDIOL (SERINOL)
1,3-Dihydroxy-2-propylamine
1,3-Dihydroxyisopropylamine
2-Aminoglycerol | [EINECS(EC#)]
208-584-0 | [Molecular Formula]
C3H9NO2 | [MDL Number]
MFCD00051487 | [Molecular Weight]
91.11 | [MOL File]
534-03-2.mol |
| Chemical Properties | Back Directory | [Appearance]
white to off-white adhering crystalline powder | [Melting point ]
52-55 °C (lit.) | [Boiling point ]
277 °C (lit.) | [density ]
1.1278 (rough estimate) | [vapor pressure ]
0.01Pa at 25℃ | [refractive index ]
1.4698 (estimate) | [Fp ]
>230 °F
| [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
DMSO (Soluble, Sonicated), Methanol (Slightly, Sonicated), Water (Slightly) | [form ]
Adhering Crystalline Powder | [pka]
12.24±0.10(Predicted) | [color ]
White to off-white | [Water Solubility ]
Soluble in water and alcohol. | [Sensitive ]
Hygroscopic | [BRN ]
635683 | [Stability:]
Hygroscopic | [InChIKey]
KJJPLEZQSCZCKE-UHFFFAOYSA-N | [LogP]
-1.82 at 20℃ | [CAS DataBase Reference]
534-03-2(CAS DataBase Reference) | [NIST Chemistry Reference]
2-Amino-1,3-propanediol(534-03-2) | [Storage Precautions]
Moisture sensitive | [EPA Substance Registry System]
534-03-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
C,Xi | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S27:Take off immediately all contaminated clothing .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3263 8/PG 2
| [WGK Germany ]
1
| [F ]
3-10-34 | [Hazard Note ]
Corrosive | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29221990 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
1,3-DIOXANE-->2-AMINO-1,3-PROPANEDIOL OXALATE-->1,3-Dioxan-5-amine,2-phenyl-(9CI)-->1,3-Propanediol, 2,2'-iminobis--->L-Serine methyl ester hydrochloride-->DIMETHYL 2-AMINOMALONATE, TECH-->2-Nitro-1,3-propanediol-->2,5-Dihydroxy-1,4-dioxane-2,5-dimethanol | [Preparation Products]
L-Serine-->Iopamidol-->2-(DiMethylaMino)propane-1,3-diol |
| Questions And Answer | Back Directory | [Chemical Properties]
2-amino-1, 3-propanediol, which carries the trivial name of serinol, is an organic compound from the group of alkanolamines.The name serinol was chosen because of the structural similarity to the amino acidserine. In extension, derivatives thereof with substituents on the carbons of serinol are also called serinols.Under standard conditions, it is a solid, which is very soluble in water. 2-amino-1, 3-propanediol is a very stable, hygroscopic and corrosive substance. | [Application]
- In biological field, the amino alcohol structure is widely found in many drugs especially antihistamines.
- Serinol is used as an intermediate for non-ionic X-ray contrast agents like iopamidol (1-N,3-N-bi's (1,3-dihydroxypropan-2-yl) -5-[(2S) -2-hydroxypropanamido] -2,4,6-triiodobenzene-1,3-di-carboxamide, which is for example distributed as iopamiro®, isovue® (both Bracco Diagnostics Inc.) or scanlux® (Sanochemia). Iopamidol is employed as a contrast agent for angiography throughout the cardiovascular system.
- Except the application in biochemical field, Serinol is used for the production of efficient anionic emulsifiers and nonionic polyethylene emulsions. It appears in personal care product formulations and show better base strength, lower neutral equivalent, and lower volatility than traditional amine emulsifiers.Furthermore, serinol constitutes a precursor for drugs dealing with pain treatment.
- The difunctionality of serinol makes it useful as raw materials in polymer applications in both water- and solvent-based to increase the solubility of other components and improve solution stability. It functions as a pigment dispersion-aid dispersant to enhance the wetting and viscosity stability.
- Serinol maintains a constant alkalinity in the boiling water flows and condensate not to form solid products which would impede line flow. It is applied for corrosion Inhibits. So it is widely employed in the preparation of water soluble cationic flocculants and ion exchange resins which adsorb solid and colloidal particles by electrostatic attraction. It is also used for water treatment, metal treatment and absorption of CO2 gas.
|
| Hazard Information | Back Directory | [Description]
2-Amino-1,3-propanediol (2-APD, also known as serinol) (534-03-2) is a high-value-added chemical that is used as a building block for the synthesis of a variety of compounds, including drugs to treat type 2 diabetes mellitus, the antibiotic chloramphenicol, immune multiple sclerosis, and a new generation of nonionic X-ray contrast agents.
| [Uses]
2-Amino-1,3-propanediol is used as an intermediate in several chemical applications and also useful for serinol and some of its derivatives. | [Preparation]
Add 20% 2-nitro-1,3-propanediol methanol solution (1250 g) to 5% palladium/carbon 3.5 g. Hydrogen (70 L, pressure 3MPa) was continuously introduced and reacted at 75°C for 5 hours to obtain serine alcohol solution. The serine alcohol solution is pumped into a high vacuum distillation vessel, vacuumed to 40 pa, and methanol is distilled for reuse. Yield = 97.2%.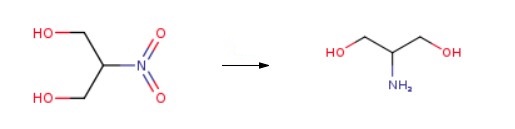 | [Biosynthesis]
Currently, the manufacture of 2-Amino-1,3-propanediol (2-APD) predominantly relies on chemical processes by utilizing fossil fuel-derived and highly explosive raw materials. In addition to chemical synthesis, its synthesis can also rely on biosynthesis. Luo et al. established an artificial biosynthetic pathway for converting glucose to 2-APD in a metabolically engineered Escherichia coli. This artificial pathway employs an engineered heterogeneous aminotransferase RtxA for diverting dihydroxyacetone phosphate (DHAP) to generate 2-APD phosphate and an endogenous phosphatase for converting it into the target product 2-APD. 2-APD-P is 2-amino-1,3-propanediol phosphate[1].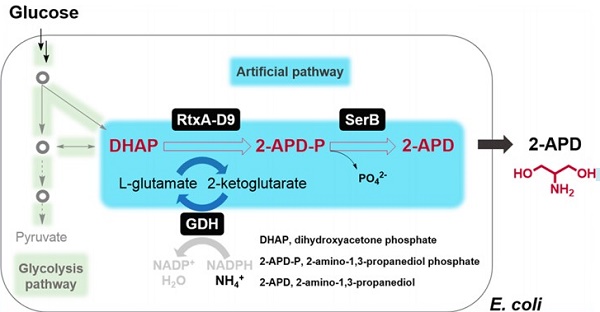
| [Origin]
In the 1970s, 2-Amino-1,3-propanediol (2-APD) was first reported to be a molecular
building block of the natural products rhizobitoxine and
dihydrorhizobitoxine produced by the legume symbiont
Rhizobium japonicum[1]. | [General Description]
Serinol activates toxin production in the attenuated cultures of Helminthosporium sacchari. Preparation of polyesters derived from serinol is reported. | [References]
[1] Luo Y, et al. An Artificial Biosynthetic Pathway for 2?Amino-1,3-Propanediol
Production Using Metabolically Engineered Escherichia coli. ACS Synthetic Biology, 2019; 8: 548–556.
|
|
|