| Identification | More | [Name]
Loperamide | [CAS]
53179-11-6 | [Synonyms]
LOPERAMIDE
4-(4-chlorophenyl)-n,n-dimethyl-alpha,alpha-diphenyl-4-hydroxy-1-piperidineb
utanamide
4-[4-Chlorophenyl]-4-hydroxy-N, N-dimethyl-α,α-diphenyl-1-piperidinebutanamide
4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidyl]-N,N-dimethyl-2,2-diphenyl-butanamide
4-(4-Chlorophenyl)-4-hydroxy-N,N-dimethyl-,-diphenyl-1-p-piperidinebutanamide
4-[4-(4-Chlorophenyl)-4-hydroxypiperidin-1-yl]-N,N-dimethyl-2,2-di(phenyl)butanamide
Tebloc
4-(4-Chlorophenyl)-4-hydroxy-N,N-dimethyl-α,α-diphenyl-1-piperidinebutyramide
4-[4-(p-Chlorophenyl)-4-hydroxypiperidino]-2,2-diphenyl-N,N-dimethylbutyramide
4-[4-Hydroxy-4-(4-chlorophenyl)-1-piperidinyl]-2,2-diphenyl-N,N-dimethylbutanamide | [EINECS(EC#)]
258-416-5 | [Molecular Formula]
C29H33ClN2O2 | [MDL Number]
MFCD00600388 | [Molecular Weight]
477.04 | [MOL File]
53179-11-6.mol |
| Hazard Information | Back Directory | [Originator]
Imodium,Janssen,UK,1975 | [Uses]
Loperamide is presently used more often as an antidiarrheal drug than as an analgesic, and
it is also included in the list of over-the-counter drugs because of its insignificant action on
the CNS. It reduces intestinal smooth muscle tone and motility as a result of binding to
intestinal opiate receptors. It is used for symptomatic treatment of severe and chronic diar�rhea of various origins. The most popular synonym for loperamide is imodium. | [Definition]
ChEBI: A synthetic piperidine derivative, effective against diarrhoea resulting from gastroenteritis or inflammatory bowel disease. | [Manufacturing Process]
23.6 parts of 2-oxo-3,3-diphenyl-tetrahydrofuranare melted at 100°C in an
oil-bath and gaseous hydrogen bromide is introduced into it during 3 hours.
The reaction mixture is cooled and triturated in benzene. The product is
filtered off, washed with petroleum ether and dried in an exsiccator, yielding
4-bromo-2,2-diphenylbutyric acid; MP 127.5%.
To a stirred suspension of 16 parts of 4-bromo-2,2-diphenylbutyric acid in 150
parts of chloroform are added dropwise 16 parts of thionyl chloride and the
whole is stirred and refluxed for 2 hours. The reaction mixture is evaporated,yielding 4-bromo-2,2-diphenyl-butyrylchloride as a residue.
60 parts of 4-bromo-2,2-diphenylbutyrylchloride are dissolved in 400 parts of
toluene and gaseous dimethylamine is introduced slowly into the solution
while cooling (temperature is kept at about 0°C). The introduction is ceased
when dimethylamine escapes from the cooler, and stirring is continued for 2
hours at ordinary temperature. The precipitated product is filtered off and
dissolved in a minimum quantity of water. The product is extracted with
chloroform. The extract is dried and evaporated. The residue solidifies on
triturating in 4-methyl-2-pentanone. The solid is filtered off and dried, yielding
dimethyl -(tetrahydro-3,3-diphenyl-2-furylidene)ammonium bromide; MP 169°
to 171.5°C.
A mixture of 6.33 parts of 4-(p-chlorophenyl)-4-piperidinol, 8 parts of sodium
carbonate, 0.2 part of potassium iodide and 240 parts of 4-methyl-2-
pentanone is distilled azeotropically. Then there are added 12.12 parts of
dimethyl-(tetrahydro-3,3-diphenyl-2-furylidene)ammonium bromide (from the
preceding step) and the whole is stirred and refluxed for about 15 hours. The
reaction mixture is filtered hot and the filtrate is evaporated.
The oily residue is dissolved in 2-propanol and to this solution is added an
excess of 2-propanol previously saturated with gaseous hydrogen chloride.
The whole is evaporated and the oily residue is warmed in diluted hydrochloric
acid solution. Upon the addition of toluene, the salt is precipitated. It is
filtered off, boiled in acetone, and filtered off again after cooling, yielding 4-
(p-chlorophenyl)-4-hydroxy-N,N-dimethyl-α,α-diphenylpiperidine-1-butyramide
hydrochloride; MP 222.1°C. | [Brand name]
Ami-29;Colifelin;Colifilm;Diareze;Dissenter;Duplibiot;Elcoman;Firtasec;Loperan;Loperin;Lopermid;Motilix;Orulop;Pf 185;Pricilone;R-18553;Regulane;Seldiar;Taguinol;Telboc;Totrtasec. | [Therapeutic Function]
Antidiarrheal | [World Health Organization (WHO)]
Loperamide, an inhibitor of intestinal peristalsis, was introduced
in 1975 for the treatment of acute and chronic diarrhoea. In many countries its use
was discouraged in young children. In late 1989, treatment of infants in Pakistan
was associated with 19 cases of paralytic ileus, 6 of which have been fatal. This
has subsequently led the major manufacturer to withdraw all drop formulations of the drug worldwide as well as the lower dose syrup forms from countries where
there is a programme for the control of diarrhoeal diseases. The WHO Control of
Diarrhoeal Diseases Programme recommends that loperamide should not be used
in children below five year of age.
(Reference: (LJJ) Letter to WHO from Johnson & Johnson, , , 21 June 1990) | [General Description]
Loperamide (Imodium) is a 4-phenylypiperidine with amethadone-like structure attached to the piperidine nitrogen. It acts as an antidiarrheal by directly binding tothe opiate receptors in the gut wall. Loperamide inhibitsacetylcholine and prostaglandin release, decreasing peristalsisand fluid secretion thus increasing the GI transit time andreducing the volume of fecal matter.Loperamide is sufficiently lipophilic to cross the blood-brain barrier, yet itdisplays no CNS-opioid effects. The reason for this is that itis actively pumped out of the brain via the P-glycoproteinpump (MDR1). Knockout mice with the P-glycoproteinpump genetically removed were given radiolabeled loperamideand sacrificed 4 hours later. The [3H]loperamideconcentrations were measured and compared with wild-typemice. A 13.5-fold increase in loperamide concentration wasfound in the brain of the knockouts. In addition, the micelacking the P-glycoprotein pump displayed pronouncedsigns of central opiate agonism. Loperamide is availableas 2-mg capsules for treatment of acute and chronic diarrhea.Recommended dosage is 4 mg initially, with 2 mgafter each loose stool for a maximum of 16 mg/d. | [Clinical Use]
Loperamide is a synthetic
opioid subjected to extensive first-pass
metabolization after oral administration. Therefore
little intact drug reaches the systemic circulation
and the central nervous system. Loperamide
has no centrally mediated analgesic efficacy,
but may have a clinically useful analgesic effect via peripheral opioid receptors . Systemic
and central opioid side effects are widely
missing. Orally administered loperamide acts locally
in the gut by inhibition of intestinal motility
and secretion . Besides the strong μ-
opioid action, calcium and calmoduline antagonism
are involved in the antidiarrheal activity.
The compound is used in doses of 4–8 mg
for the treatment of acute and chronic diarrhea
and for the management of colostomies and
ileostomies . Adverse effects include nausea,
dry mouth, dizziness. High doses can induce
toxic megacolon and paralytic ileus. The compound
has no abuse and dependence potential
and is meanwhile available as over the counter
(OTC) product . | [Synthesis]
Loperamide, 1-(4-chlorophenyl)-4-hydroxy-N,N-dimethyl-|á,|á-diphenyl-1-
piperidinebutyramide (3.1.55), proposed here as an analgesic, is synthesized by the alky�lation of 4-(4-chlorophenyl)-4-hydroxypiperidine (3.1.50) using N,N-dimethyl(3,3-
diphenyltetrahydro-2-furylidene)ammonium bromide (3.1.54) in the presence of a base.
The 4-(4-chlorophenyl)-4-hydroxypiperidine (3.1.50) is synthesized by reacting
1-benzylpiperidine-4-one (3.1.48) with 4-chlorophenylmagnesiumbromide, followed by
debenzylation of the product (3.1.49) by hydrogenation using a palladium on carbon
catalyst.
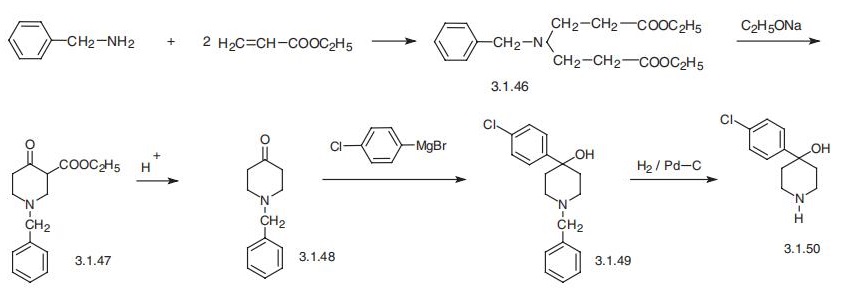
The starting 1-benzylpiperidin-4-one (3.1.48) is synthesized by Dieckmann intermolec�ular condensation of N-benzyl-N,N-bis-(|?-carboethoxyethyl)amine (3.1.46), which is easily
formed by reaction of benzylamine with ethyl acrylate to give 1-benzyl-3-carboethoxy�piperidine-4-one (3.1.47) followed by acidic hydrolysis and thermal decarboxylation.
N,N-Dimethyl-(3,3-diphenyltetrahydro-2-furyliden)ammonium bromide (3.1.54) is syn�thesized from diphenylacetic acid ethyl ester, which is reacted with ethylene oxide in the
presence of sodium hydroxide, giving 2,2-diphenylbutyrolactone (3.1.51). Reacting this
with hydrogen bromide in acetic acid opens the lactone ring, forming 2,2-diphenyl-4-bro�mobutyric acid (3.1.52). This transforms into acid chloride (3.1.53) using thionyl chloride,
which cyclizes upon further treatment with an aqueous solution of dimethylamine, thus
forming the desired N,N-dimethyl-(3,3-diphenyltetrahydro-2-furyliden)ammonium bro�mide (3.1.54). Reacting this with 4-(4-chlorphenyl)-4-hydroxypiperidine (3.1.50) gives the
desired loperamide (3.1.55) [34¨C36].

| [Veterinary Drugs and Treatments]
Loperamide is used as a GI motility modifier in small animals. Use
in cats is controversial and many clinicians do not recommend using
in cats. |
|
|