| Identification | More | [Name]
Acrylic anhydride | [CAS]
2051-76-5 | [Synonyms]
2-PROPENOIC ACID ANHYDRIDE
ACRYLIC ACID ANHYDRIDE
ACRYLIC ANHYDRIDE
Acyrlic acid anhydride
PROPENOIC ANHYDRIDE
Diacrylic anhydride | [EINECS(EC#)]
218-128-2 | [Molecular Formula]
C6H6O3 | [MDL Number]
MFCD00048146 | [Molecular Weight]
126.11 | [MOL File]
2051-76-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
-20 °C | [Boiling point ]
85-86°C 17mm | [density ]
1,094 g/cm3 | [Fp ]
77°C | [storage temp. ]
2-8°C | [solubility ]
Chloroform (Sparingly), Ethyl Acetate (Slightly) | [form ]
clear liquid | [color ]
Colorless to Almost colorless | [Stability:]
Moisture sensitive | [InChI]
InChI=1S/C6H6O3/c1-3-5(7)9-6(8)4-2/h3-4H,1-2H2 | [InChIKey]
ARJOQCYCJMAIFR-UHFFFAOYSA-N | [SMILES]
C(=O)(C=C)OC(=O)C=C | [CAS DataBase Reference]
2051-76-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Risk Statements ]
R14:Reacts violently with water.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
3265 | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
2916199590 |
| Questions And Answer | Back Directory | [Description]
Acrylic anhydride is used to prepare specialty acrylate, acrylamide monomers. Forms cyclic anhydrides on polymerization and does not produce crosslinks in polymers. It is used in the preparation of acrylic resin. And it is also used in organic synthesis. For example: it can react with adamantan-1-ol to get acrylic acid adamantan-1-yl ester. This reaction needs catalytic agent acidic catalyst and solvent cyclohexane by heating. The yield is 95%.
|
| Hazard Information | Back Directory | [Uses]
Acrylic anhydride can be used to prepare specialty acrylate, acrylamide monomers or acrylic resin. It forms cyclic anhydrides on polymerization and can not be used as a crosslinker. | [Synthesis]
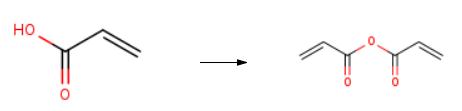
The synthesis of Acrylic anhydride is as follows:
In a 3-liter, four-necked glass reactor provided with a thermometer and a stirrer were placed, in a dry air atmosphere, 1,240 g of methylene chloride, 1.08 g (0.5% by mass) of Sumilizer GM, 0.65 g (0.3% by mass) of Sumilizer TP-D, 0.65 g (0.3% by mass) of Sumilizer WX-R, 216 g (3.0 mol) of acrylic acid and 264 g (1.5 mol) of benzenesulfonyl chloride. The mixture was cooled to 5°C. Then, 304 g (3.0 mol, 1 equivalent relative to the acids generated from benzenesulfonyl chloride) of triethylamine was dropwise added in 2 hours with the temperature of the reaction mixture being controlled at 30°C or lower. After the completion of the dropwise addition, stirring was made for 1 hour with the same temperature being kept. The reaction mixture was analyzed by GC, which indicated that the conversion of acrylic acid was 99%. After the completion of the reaction, 375 g of water was added to the reaction mixture to wash the reaction mixture. The reaction mixture was further washed twice each time with 563 g of water, after which distillation was conducted to remove methylene chloride. The yield of the acrylic acid anhydride obtained was 172 g and 91% and its purity by GC analysis was 99%, and no high-molecular by-product was detected.
 |
|
|