| Identification | More | [Name]
Ezetimibe | [CAS]
163222-33-1 | [Synonyms]
1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-hydroxy-propyl]-4-(4-hydroxyphenyl)-azetidin-2-one
EZETIMIBE
ZETIA
Ezitimibe&Int
EzetimibeC24H21F2N03
(3R,4S)-1-(4-Fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-2-azetidinone, Sch-58235
1-(4-Flurophenyl)-(3R)-3-(4-flurophenyl)-(3S)-hydroxypropyl-(4S)-(4-hydroxyphenyl)-2-azetidinone
Ezetimide
SCH-58235 | [EINECS(EC#)]
682-606-0 | [Molecular Formula]
C24H21F2NO3 | [MDL Number]
MFCD00937872 | [Molecular Weight]
409.43 | [MOL File]
163222-33-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
164-166°C | [alpha ]
D22 -33.9° (c = 3 in methanol) | [Boiling point ]
654.9±55.0 °C(Predicted) | [density ]
1.334±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 15 mg/ml) | [form ]
powder | [pka]
9.72±0.30(Predicted) | [color ]
White or off-white | [Usage]
An antihyperlipoproteinemic. A Cholesterol absorption inhibitor | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. | [InChI]
InChI=1S/C24H21F2NO3/c25-17-5-1-15(2-6-17)22(29)14-13-21-23(16-3-11-20(28)12-4-16)27(24(21)30)19-9-7-18(26)8-10-19/h1-12,21-23,28-29H,13-14H2/t21-,22+,23-/m1/s1 | [InChIKey]
OLNTVTPDXPETLC-XPWALMASSA-N | [SMILES]
N1(C2=CC=C(F)C=C2)[C@H](C2=CC=C(O)C=C2)[C@@H](CC[C@@H](C2=CC=C(F)C=C2)O)C1=O | [CAS DataBase Reference]
163222-33-1(CAS DataBase Reference) | [EPA Substance Registry System]
2-Azetidinone, 1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-, (3R,4S)- (163222-33-1) |
| Safety Data | Back Directory | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [HS Code ]
29337900 | [Hazardous Substances Data]
163222-33-1(Hazardous Substances Data) |
| Questions And Answer | Back Directory | [Description]
Ezetimibe is ananti-hyperlipidemic drug used for lowering the plasma cholesterol levels. It is indicated as an adjunctive therapy to diet for the reduction of high-level total-C, LDL-C, and ApoB in patients suffering primary (heterozygous familial and non-familial) hypercholesterolemia. It is also used in combination therapy with HMG-CoA reductase inhibitors.Ezetimibe does not inhibit the cholesterol synthesis in the liver, or increase bile acid excretion.It takes effect throughacting at the brush border of the small intestine and inhibiting the absorption of cholesterol, further leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in clearance of blood cholesterol.
| [Indications and Usage]
Ezetimibe is a new form of selective cholesterol absorption inhibitor developed in a collaboration between Schering-Plough Co. and Merck Co. This drug is the first cholesterol absorption selective inhibitor to be approved for sale by the American FDA. Its commercial name is Ezetrol.
This drug can be used alone or in combination with HMG-CoA reductase inhibitors (statins) to treat primary (heterozygous familial and non-familial) hypercholesterolemia, homozygous familial hypercholesterolemia (HoFH), homozygous viremia (or phytosterolemia).
| [Mechanisms of Action]
Ezetimibe’s mechanisms of action are different from those of other lipid-lowering drugs (such as statins, cholic acid chelating agents, phenoxy acid derivatives, and plant sterols). This drug binds with the surface proteins on the brush border membrane vesicles of the small intestine (relative molecular mass 145x10^3) to inhibit the small intestine’s absorption of cholesterol in food and bile, thus decreasing the cholesterol content in serum and the liver. Ezetimibe is different from bile acid sequestrants because it does not affect the absorption of cholesterol esters, other steroids (such as bezoar and cholic acid), three triacylglycerol, and fat-soluble vitamins. Its effects are unrelated to whether or not acetyl coenzyme A- cholesterol acetyltransferase (ACAT) is inhibited or whether or not the LDL receptor (scavenger receptor) is expressed. After Ezetimibe is absorbed and binds with glucuronic acid in the liver, it undergoes enterohepatic circulation and almost exclusively targets small intestine mucosa cells.
| [References]
https://en.wikipedia.org/wiki/Ezetimibe
https://pubchem.ncbi.nlm.nih.gov/compound/Ezetimibe#section=Top
https://www.drugbank.ca/drugs/DB00973
Davidson, Michael H, et al. "Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia ☆." Journal of the American College of Cardiology 40.12(2002):2125.
Sudhop, T, et al. "Inhibition of intestinal cholesterol absorption by ezetimibe in humans. " Circulation 106.15(2002):1943-8.
|
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Originator]
Schering-Plough (USA) | [Uses]
A cholesterol transport inhibitor that binds to NPC1L1 | [Uses]
An antihyperlipoproteinemic. A Cholesterol absorption inhibitor | [Uses]
antibacterial | [Uses]
Ezetimibe (9) was approved as the first hypolipidemic
drug to act by blocking the absorption of dietary cholesterol.
This drug was discovered by Schering-Plough and is codeveloped
and co-marketed by Merck and Schering-Plough
for the treatment of hypercholesterolemia and also two less
common forms of hyperlipidemia: homozygous familial
hypercholesterolemia and homozygous sitosterolemia. | [Uses]
For use as adjunctive therapy to diet for the reduction of elevated total-C, LDL-C, and Apo B in patients with primary (heterozygous familial and non-familial) hypercholesterolemia. | [Definition]
ChEBI: Ezetimibe is a beta-lactam that is azetidin-2-one which is substituted at 1, 3, and 4 by p-fluorophenyl, 3-(p-fluorophenyl)-3-hydroxypropyl, and 4-hydroxyphenyl groups, respectively (the 3R,3'S,4S enantiomer). It has a role as an anticholesteremic drug, an antilipemic drug and an antimetabolite. It is a member of azetidines, an organofluorine compound and a beta-lactam. | [Brand name]
Zetia (Merck/Schering-Pough);Ezetrol. | [Biological Functions]
Ezetimibe lowers plasma cholesterol levels by inhibiting the absorption of cholesterol at the brush border of the small intestine.
Specifically, it has been proposed to bind to a specific transport protein located in the wall of the small intestine, resulting in a reduction
of cholesterol transport and absorption. Ezetimibe appears to be selective in its actions in that it does not interfere with the
absorption of triglycerides, lipid-soluble vitamins or other nutrients. The decreased absorption of cholesterol eventually leads to enhanced receptor-mediated LDL uptake similar to that seen with bile acid sequestrants and HMGRIs. When used as
monotherapy, the decreased absorption of cholesterol causes a compensatory increase in cholesterol biosynthesis. This is similar to
that described for bile acid sequestrants and is insufficient to override the overall LDL lowering effects of ezetimibe. | [General Description]
Ezetimibe, (3R,4S)-1-(4-fluorophenyl)-3-((3S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)-2-azetidinone (Zetia), is an antihyperlipidemicagent that has usefulness in lowering cholesterol levels. Itacts by decreasing cholesterol absorption in the intestine byblocking the absorption of the sterol at the Brush boarder.Specifically, the -lactam binds to the Niemann-Pick C1-Like 1 (NPC1L1) protein on the gastrointestinal tract that isresponsible for cholesterol absorption. Although it may beused alone, it is marketed as a combination product withsimvastatin under the trade name Vytorin. | [Biochem/physiol Actions]
Ezetimibe is a non statin drug that reduces intestinal cholesterol absorption. In addition, it also has an ability to reduce the risk of cardiovascular events in patients who had had an acute coronary syndrome and whose low-density lipoprotein (LDL) cholesterol values were within guideline recommendations. | [Pharmacokinetics]
Ezetimibe is administered orally; however, its absolute bioavailability cannot be determined because of its aqueous insolubility and the
lack of an injectable formulation. Based on area under the curve values, the oral absorption ranges from 35 to 60%. Mean peak
concentrations of the active glucuronidated metabolite are reached within 1 to 2 hours. Both ezetimibe and its glucuronide conjugate are
extensively bound (>90%) to plasma proteins. The relative plasma concentrations of ezetimibe and its glucuronide conjugate range from
10 to 20% and from 80 to 90%, respectively. Both compounds have a long half-life of approximately 22 hours. The coadministration of
food with ezetimibe has no effect on the extent of absorption. | [Clinical Use]
Ezetimibe is indicated as monotherapy or in combination with an HMGRI for the reduction of elevated total cholesterol, LDL cholesterol,
and apoB in patients with primary (heterozygous familial and nonfamilial) hypercholesterolemia. When used as monotherapy, ezetimibe
reduces LDL cholesterol by approximately 18%. When used in combination therapy with an HMGRI, LDL levels are reduced by 25 to 65%
depending on the dose of the HMGRI inhibitor. Ezetimibe also is indicated for homozygous familial hypercholesterolemia in combination
with either atorvastatin or simvastatin and for homozygous familial sitosterolemia. All indications are for patients who have not
responded to diet, exercise, and other nonpharmacological methods. | [Side effects]
Ezetimibe generally is well tolerated. The most common adverse effects are listed above. Whenever ezetimibe is used in combination
with an HMGRI, the incidence of myopathy or rhabdomyolysis does not increase above that seen with HMGRI monotherapy. | [Synthesis]
The synthesis of ezetimibe (9) begins with the one-step
diastereoselective and practical synthesis of the trans |?-
lactam from commercially available (S)-3-hydroxy-|?-lactone
(92). Lactam 95 was obtained by generation of a dianion of
lactone 92 with LDA in THF followed by addition of the
imine and N,N?ˉ-dimethylpropyleneurea (DMPU) to give
predominately adduct 93 (93:94 = 79:21). However,
intermediate 93 and 94 did not cyclize to their respective
lactams due to formation of stable lithium aggregates.Addition of lithium chloride/DMF was employed to cyclize
the intermediates into trans-lactam 95 as the major product
(trans:cis = 95:5) in a one-pot process from 92 in 64%
yield. The 95:5 ratio of compound 95 was oxidatively
cleaved with NaIO4 to give aldehyde 96. Mukaiyama aldol
condensation was adopted to elaborate the 4-fluorophenylpropyl
side chain to give alcohol 98. Without
isolation, the reaction mixture was subjected to dehydration
using p-TSA to give enone 99 in 75% yield from compound
96. Reduction of the double bond in 99 with Wilkinson?ˉs
catalyst yielded ketone 100, which was subjected to the
highly enantioselective CBS reduction to give alcohol 101
with a 98:2 selectivity of S:R at the benzylic position.
Catalytic hydrogenation of compound 101 gave ezetimibe
(9) in 79% yield. Alternatively, a palladium-catalyzed
double reduction in EtOAc/MeOH of both the double bond
and the benzyl protecting group in enone 99 produced free
phenol 107 in 90% yield. A three-step one-pot procedure
was subsequently developed to transform 107 into ezetimibe
(9) in 79% yield. That is, free phenol 107 was protected in
situ as its TMS ether using BSU followed by a highly
selective CBS reduction of the ketone group to give the desired alcohol in 97% ee. The TMS group was removed
during acidic workup to give ezetimibe (9). A more
convergent approach to this drug was also developed by
preparing the (S)-hydroxy side chain before the ring
construction. Therefore, p-fluorobenzoylbutyric acid
(102) was reacted with pivaloyl chloride and the acid
chloride thus obtained was acylated with chiral auxiliary 103
to give the corresponding amide. The ketone group in the
amide was reduced with (R)-MeCBS/BH3-THF (104) in the
presence of p-TSA to give desired alcohol 105 in high yield
(99%) and stereoselectivity (96 % d.e.). Chiral alcohol
105 was then mixed with the imine in the presence of
TMSCl and DIPEA to protect the alcohols as TMS ethers.
In the same pot, TiCl4 was added to catalyze the
condensation reaction and gave compound 106 in 65% yield.
Compound 106 was reacted with TBAF and a fluoridecatalyzed
cyclization took place to give the corresponding
lactam. Finally, the TMS protecting group was removed
under acidic conditions to give ezetimibe (9) in 91% yield
over two steps.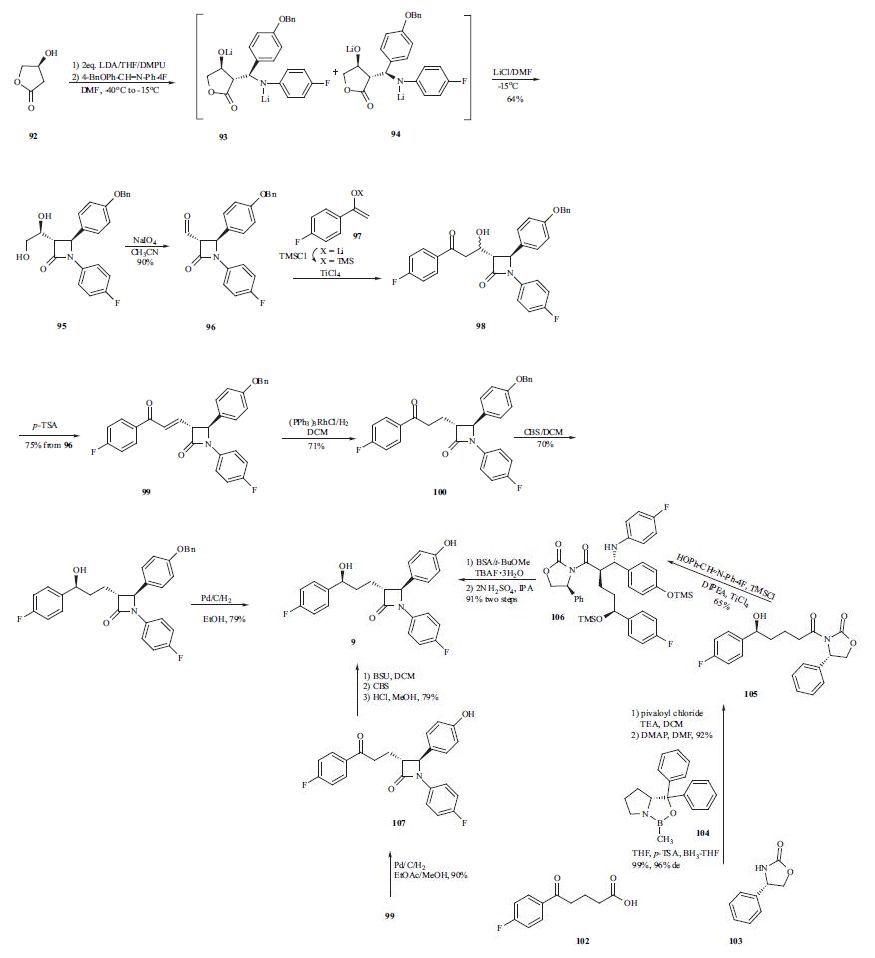
| [target]
MEK | ERK | [Drug interactions]
Potentially hazardous interactions with other drugs
Ciclosporin: concentration of both drugs possibly
increased.
Lipid lowering agents: avoid with fibrates;
concentration of rosuvastatin increased - reduce
rosuvastatin dose. | [Metabolism]
Following oral administration, ezetimibe is rapidly and extensively metabolized in the intestinal wall and the liver to its active metabolite,
a corresponding phenol glucuronide. This glucuronide is reexcreted in the bile back to its active site. A small amount (<5%) of ezetimibe
undergoes oxidation to covert the benzylic hydroxyl group to a ketone; however, ezetimibe does not appear to exert any significant effect
on the activity of CYP450 enzymes. |
|
|