| Identification | More | [Name]
Pancuronium bromide | [CAS]
15500-66-0 | [Synonyms]
1,1'-[(2b,3a,5a,16b,17b)-3,17-bis(acetyloxy)androstane-2,16-diyl]bis(1-methylpiperidinium) dibromide
1,1'-[(2BETA,3ALPHA,5ALPHA,16BETA,17BETA)-3,17-BIS(ACETYLOXY)ANDROSTANE-2,16-DIYL]BIS[1-METHYLPIPERIDINIUM]DIBROMIDE
1,1'-(3A,17B-DIHYDROXY-2B,5A-ANDROSTAN-2B,16B-YLENE) BIS[1-METHYLPIPERIDINIUM] DIACETATE DIBROMIDE
PANCURONIUM BROMIDE
PANCURONIUM DIBROMIDE
1,1’-(3,17-bis(acetyloxy)androstane-2,16-diyl)bis(1-methylpiperidinium)dibro
1,1’-(3alpha,17beta-dihydroxy-5alpha-androstan-2beta,16beta-ylene)bis[1-methyl
1,1’-[3alpha,17beta-bis(acetyloxy)-5alpha-androstane-2beta,16beta-diyl]bis[1-m
16-diyl)bis(1-methyl-ostane-dibromide
2beta,16beta-dipiperidino-5alpha-androstane-3alpha,17beta-dioldiacetatedimetho
3-alpha,17-beta-diacetoxy-2-beta,16-beta-dipiperidino-5-alpha-androstanedime
3-alpha,17-beta-diacetoxy-2-beta,16-beta-dipiperidino-5-alpha-androstanedimeth
3alpha,17beta-diacetoxy-2beta,16beta-dipiperidino-5alpha-androstanedimethobrom
5alpha-androstan-3alpha,17beta-diol,2beta,16beta-dipipecolinio-,dibromide,
5alpha-androstan-3alpha,17beta-diol,2beta,16beta-dipipecolinio-,dibromide,di
diacetate
ethylpiperidinium]dibromide
mioblock
na97
orgna97 | [EINECS(EC#)]
239-532-5 | [Molecular Formula]
C35H60Br2N2O4 | [MDL Number]
MFCD00079223 | [Molecular Weight]
732.67 | [MOL File]
15500-66-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
215° | [Boiling point ]
~100°C | [density ]
~1, mp: 0°C | [storage temp. ]
2-8°C | [solubility ]
Very soluble or freely soluble in water, very soluble in methylene chloride, freely soluble in ethanol (96 per cent). | [form ]
Off-white solid | [color ]
Clear, colorless solution | [Odor]
Odorless | [Water Solubility ]
water: 100mM | [Merck ]
13,7077 | [BRN ]
4226892 | [InChIKey]
NPIJXCQZLFKBMV-LQDGISGANA-L | [CAS DataBase Reference]
15500-66-0(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed. | [RIDADR ]
UN 2811 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
TN4930000
| [F ]
10-21-33 | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
2933399090 | [Hazardous Substances Data]
15500-66-0(Hazardous Substances Data) | [Toxicity]
LD50 in mice (mg/kg): 0.047 i.v.; 0.152 i.p.; 0.167 s.c.; 21.9 orally; in rats, rabbits: 0.153, 0.016 i.v. (Buckett, 1968) |
| Hazard Information | Back Directory | [Description]
Pancuronium bromide, a bisquaternary amine and the first steroid N MBA used clinically, was developed by Savege and Hewitt and marketed in 1964. The intubating dose is 0.1mgkg–1, which takes 3-4min to reach its maximum effect. The clinical duration of action of the drug is long, especially in the presence of potent inhalational agents or renal dysfunction, as 60% of a dose of the drug is excreted unchanged through the kidneys. I t is also deacetylated in the liver; some of the metabolites have neuromuscular blocking properties.
Pancuronium does not stimulate histamine release; however, it has direct vagolytic and sympathomimetic effects which may cause tachycardia and hypertension. It slightly inhibits plasma cholinesterase and therefore potentiates any drug metabolised by this enzyme, such as suxamethonium and mivacurium. | [Chemical Properties]
Light Beige Solid | [Originator]
Pavulon,Organon-Teknika ,UK,1968 | [Uses]
A potent steroidal neuromuscular blocking agent; muscle relaxant. | [Uses]
Pancuronium is a steroid compound that does not possess hormonal activity. It is used in
anesthesiology as a myorelaxant, causing prolonged muscle relaxation during surgical
interventions of the thoracic and abdominal cavities, in proctology, ophthalmology, orthopedic
practice, and in heart surgeries. A synonym of this drug is pavulon. | [Uses]
progestinantineoplastic | [Definition]
ChEBI: A bromide salt consisting of two bromide ions and one pancuronium dication. | [Manufacturing Process]
A solution of 2α,3α,16α,17α-diepoxy-17β-acetoxy-5α-androstane (25 grams), prepared from 3,17-diacetoxy-5α-androstane-2,16-diene (Chem. Abs. 1960, 54, 8908) by treatment with m-chlor-perbenzoic acid, in piperidine (120 ml) and water (40 ml) was boiled under reflux for 5 days, the solution was concentrated and the product precipitated by the addition of water. The solid was collected, dissolved in dilute hydrochloric acid, filtered to give a clear solution and precipitated by the addition of sodium hydroxide solution. Crystallization from acetone gave 2β,16β-bis-piperidino-5α-androstan-3α-ol17-one (18.9 grams), MP 179-185°C.
A solution of sodium borohydride (8 grams) in water (16 ml) was added to a stirred solution of 2β,16β-bis-piperidino-5α-androstan-3α-ol-17-one (17 grams) in tetrahydrofuran (70 ml) and methanol (30 ml) and the solution stirred at room temperature for 16 hours. The product was precipitated by the addition of water, filtered off, dried, and crystallized from acetone to give the diol (14.9 grams).
A solution of the piperidino-diol (9 grams) in acetic anhydride (18 ml) was heated at 90°C for 1 hour, the solution cooled, excess acetic anhydride
destroyed by the careful addition of water, and the resulting solution carefully made alkaline with 2 N caustic soda solution to precipitate a solid product. The solid was dried, extracted with n-hexane and the solution filtered free of insoluble material before percolation down a column (4 x 1'' diameter) of alumina. Elution with n-hexane gave a fraction (4.2 grams) which was crystallized twice from ether to give the diacetate, MP 176°-180°C.
Methyl bromide (17 grams) was added to a solution of the bispiperidinodiacetate (4 grams) in methylene chloride (10 ml) and the resulting solution allowed to stand at room temperature for 4 days. The solution was evaporated to dryness, the residue triturated with ether, and filtered to give the bis-methobromide (5.2 grams), MP 206°C. Recrystallization from acetonemethylene chloride gave material MP 214°-217°C.
| [Brand name]
Pavulon (Organon). | [Therapeutic Function]
Muscle relaxant | [Biological Functions]
Pancuronium bromide (Pavulon) is a synthetic bisquaternary
agent containing a steroid nucleus (amino
steroid), as denoted by the -curonium suffix. It is five
times as potent as d-tubocurarine. Unlike d-tubocurarine,
it does not release histamine or block ganglionic
transmission. Like d-tubocurarine, it has a moderately
long onset (2.9 minutes) and duration of action (110
minutes). Pancuronium and its metabolite are eliminated
in the urine. | [General Description]
Although pancuronium bromide,2 ,16 -dipiperidino-5 -androstane-3 ,17 -diol diacetatedimethobromide (Pavulon), is a synthetic product, it isbased on the naturally occurring alkaloid malouetine, found inarrow poisons used by primitive Africans. Pancuronium bromideacts on the nicotinic receptor and in the ion channel,inhibiting normal ion fluxes.
This blocking agent is soluble in water and is marketed inconcentrations of 1 or 2 mg/mL for intravenous administration.It is a typical nondepolarizing blocker, with a potencyapproximately 5 times that of (+)-tubocurarine chloride anda duration of action approximately equal to the latter. Studiesindicate that it has little or no histamine-releasing potential organglion-blocking activity and that it has little effect on thecirculatory system, except for causing a slight rise in thepulse rate. As one might expect, ACh, anticholinesterases,and potassium ion competitively antagonize it, whereas itsaction is increased by inhalation anesthetics such as ether,halothane, enflurane, and methoxyflurane. The latter enhancementin activity is especially important to the anesthetistbecause the drug is frequently administered as anadjunct to the anesthetic procedure to relax the skeletal muscle.Perhaps the most frequent adverse reaction to this agentis occasional prolongation of the neuromuscular block beyondthe usual time course, a situation that can usually becontrolled with neostigmine or by manual or mechanical ventilation, since respiratory difficulty is a prominent manifestationof the prolonged blocking action. | [Biological Activity]
Nicotine (neuromuscular) antagonist. Skeletal muscle relaxant. | [Biochem/physiol Actions]
Aminosteroidal neuromuscular blocking agent; skeletal muscle relaxant | [Mechanism of action]
Pancuronium bromide is a long-acting bisquaternary aminosteroid, non-depolarising neuromuscular blocking drug (NMBD). It competitively inhibits nicotinic acetylcholine receptors at the neuromuscular junction by blocking acetylcholine binding. Like other non-depolarising NMBDs, Pancuronium bromide is a competitive inhibitor of nicotinic acetylcholine (ACh) receptor post-function; under normal conditions, nicotinic acetylcholine receptor post-function in skeletal muscle is a ligand-gated ion channel, which binds to ACh and allows the passage of sodium ions, thereby inducing a depolarisation of the cell membrane leading to skeletal muscle contraction. The nicotinic ACh receptor has five subunits, and the α-subunit is the binding site for ACh and NMBD.Pancuronium bromide contains an acetylcholine-like molecule that facilitates binding to the α-subunit. Binding of pancuronium to at least one of the alpha subunits results in a conformational change in the ACh receptor, which keeps the ion channel closed, preventing ion passage and depolarisation. In addition, pancuronium bromide is a larger molecule compared to acetylcholine and may therefore physically block ion channels, preventing ion passage; this acetylcholine receptor blocking mechanism becomes more important as the number of molecules increases. In addition, it has a slight vagal function, resulting in increased heart rate but no ganglionic paralysing (i.e., ganglion-blocking) activity. | [Pharmacology]
Pancuronium bromide, a bisquaternary amine and the first steroid NMBA
used clinically, was developed by Savege and Hewi and marketed in 1964.
The intubating dose is 0.1 mg kg–1, which takes 3–4min to reach its maximum
effect. The clinical duration of action of the drug is long,
especially in the presence of potent inhalational agents or renal dysfunction,
as 60% of a dose of the drug is excreted unchanged through the kidneys. I t is
also deacetylated in the liver; some of the metabolites have neuromuscular
blocking properties.
Pancuronium does not stimulate histamine release; however, it has direct
vagolytic and sympathomimetic effects which may cause tachycardia and
hypertension. It slightly inhibits plasma cholinesterase and therefore
potentiates any drug metabolised by this enzyme, such as suxamethonium
and mivacurium. | [Clinical Use]
As indicated, the principal use of pancuronium bromideis as an adjunct to anesthesia, to induce relaxation of skeletalmuscle, but it is also used to facilitate the managementof patients undergoing mechanical ventilation. Only experiencedclinicians equipped with facilities for applyingartificial respiration should administer it, and the dosageshould be adjusted and controlled carefully. | [Side effects]
Common side effects of Pancuronium bromide include increased salivation, skeletal muscle weakness, drooling, rash, bronchospasm, flushing, redness, low blood pressure, high blood pressure, and rapid heartbeat. | [Safety Profile]
A deadly poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx and Brí. | [Safety Profile]
A human poison by intravenous route. Poison experimentally by intravenous, subcutaneous, and intraperitoneal routes. Human systemic effects by intravenous route: chronic pulmonary edema, hemorrhage. Experimental reproductive effects. When heated to decom | [Synthesis]
Pancuronium, 1,1�-(3|á,17|?-diacetoxy-5|á-androstan-2|?,16|?-ylene)-bis-
(1-methylpiperidinium) dibromide (15.1.8), is synthesized from 3,17-bis-(acetoxy)-2,16-
5|á-androstane. Oxidation with 3-chloroperbenzoic acid gives the bis-epoxy compound
(15.1.5), the reaction of which with piperidine and subsequent hydrolysis gives an
aminoketone (15.1.6). The keto group of the resulting compound (15.1.6) is reduced by
sodium borohydride to hydroxyl group, giving the bis-aminoalcohol (15.1.7), subsequent
acetylation of which by acetic anhydride and alkylation of both nitrogen atoms by methylbromide
give the desired pancuronium (15.1.8).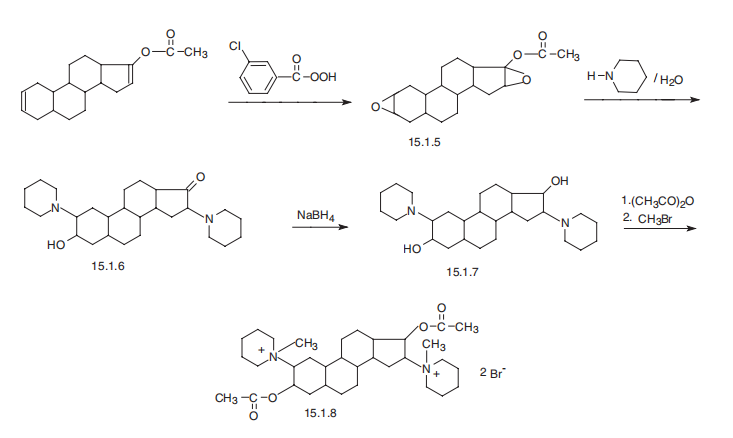
| [Veterinary Drugs and Treatments]
Pancuronium is indicated as an adjunct to general anesthesia to
produce muscle relaxation during surgical procedures or m | [Drug interactions]
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced muscle relaxant effect.
Anti-arrhythmics: procainamide enhances muscle
relaxant effect.
Antibacterials: effect enhanced by aminoglycosides,
clindamycin, polymyxins and piperacillin.
Antiepileptics: muscle relaxant effects antagonised by
carbamazepine; effects reduced by long-term use of
fosphenytoin and phenytoin but might be increased
by acute use.
Botulinum toxin: neuromuscular block enhanced
(risk of toxicity). | [Metabolism]
A small proportion of pancuronium is metabolised in the
liver to metabolites with weak neuromuscular blocking
activity.
It is largely excreted in urine as unchanged drug and
metabolites; a small amount is excreted in bile. | [Purification Methods]
The bromide forms odourless crystals with a bitter taste which are purified through acid-washed Al2O3 and eluted with isoPrOH/EtOAc (3:1) to remove impurities (e.g. the monomethobromide) and eluted with isoPrOH to give the pure dibromide which is recrystallised from CH2Cl2/Me2CO or isoPrOH/Me2CO. It is soluble in H2O (10%) and CHCl3 (3.3%) at 20o. It is a non-depolarising muscle relaxant. [Buckett et al. J Med Chem 16 1116 1973.] |
|
|