| Identification | More | [Name]
Imatinib | [CAS]
152459-95-5 | [Synonyms]
AKOS 91378
IMA-3
IMATINIB
IMATINIB-D3
IMATINIB 99+%
Benzamide, 4-[(4-methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-
Imatinib Mesilate
4-[(4-Methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide methanesulfonate
Imatinib mesylate
4-(4-METHYL-PIPERAZIN-1-YLMETHYL)-N-[4-METHYL-3-(4-PYRIDIN-3-YL-PYRIMIDIN-2-YLAMINO)-PHENYL]-BENZAMIDE
Imatinib (4-[(4-Methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide
Imatinib free base | [EINECS(EC#)]
604-855-6 | [Molecular Formula]
C29H31N7O | [MDL Number]
MFCD05662257 | [Molecular Weight]
493.6 | [MOL File]
152459-95-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
208-210°C (dec.) | [density ]
1?+-.0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Store in freezer, under -20°C | [solubility ]
DMSO (Slightly, Heated), Methanol (Slightly, Heated) | [form ]
Solid | [pka]
pKa1 8.07; pKa2 3.73; pKa3 2.56; pKa4 1.52(at 25℃) | [color ]
White to Pale Beige | [Merck ]
14,4902 | [CAS DataBase Reference]
152459-95-5(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Orange Solid | [Definition]
ChEBI: Imatinib is a benzamide obtained by formal condensation of the carboxy group of 4-[(4-methylpiperazin-1-yl)methyl]benzoic acid with the primary aromatic amino group of 4-methyl-N(3)-[4-(pyridin-3-yl)pyrimidin-2-yl]benzene-1,3-diamine. Used (as its mesylate salt) for treatment of chronic myelogenous leukemia and gastrointestinal stromal tumours. It has a role as an apoptosis inducer, a tyrosine kinase inhibitor and an antineoplastic agent. It is a N-methylpiperazine, a member of pyridines, a member of benzamides, an aromatic amine and a member of pyrimidines. It is functionally related to a benzamide. | [Indications]
Bcr-Abl inhibitor imatinib (Gleevec(R), Novartis) was approved in 2001 by the FDA. Although fasudil was approved in 1995, imatinib is widely perceived as the first approved SMKI mainly owing to the fact that fasudil's kinase inhibitory mechanism was unknown at the time of approval, and efforts to gain approval of fasudil have been unsuccessful in the United States and Europe.
The field of kinase inhibitor development has evolved rapidly since the approval of imatinib. Some of the key discoveries and events include (i) the discovery of MAPK/ERK inhibitors, for example, CI-1040 (PD184352), as the first series of type III inhibitors in 2003; (ii) the approval of first dual tyrosine kinase and serine/threonine kinase inhibitor sorafenib in 2005; (iii) the description of the first series of allosteric type IVkinase inhibitor, that is,GNF-2 and analogues that inhibit Bcr–Abl through an allosteric non-ATP-competitivemode, by Gray and coworkers in 2006; (iv) the approval of the first type III inhibitor trametinib in 2013; (v) the approval of the first covalent kinase inhibitors, afatinib and ibrutinib, in 2013; and (vi) the approval of the first lipid kinase inhibitor, that is, the PI3K inhibitor idelalisib, in 2014.
By December 2016, kinase inhibitor drug discovery can leverage the structures of over 200 human kinases and 5000 kinases of all types, over 1 million publications, clinical data from more than 200 molecules currently in phase I–III trials, and post-marketing results from the approved 38 drugs. | [Indications]
Imantinib mesylate (Gleevec) is a rationally designed
inhibitor of the tumor-specific bcr-abl kinase. The
Philadelphia chromosome, present in nearly all patients
with chronic myelogenous leukemia (CML), is produced
by a chromosomal rearrangement linking the bcr
and the abl genes. The bcr-able kinase is therefore a
unique drug target in leukemic cells, and imantinib selectively
and potently inhibits this kinase. Remissions in
CML patients are achieved with high frequency and
very low toxicity, and this compound may become a
front-line agent for treating this cancer. Unfortunately,
drug resistance has already been observed in the clinic
as a result of mutations in the bcr-abl kinase, and this
magic bullet does not appear to be curative for CML
patients. Extension of the use of imantinib to other tumor
types with overexpression of c-kit kinase or
platelet-derived growth factor kinase is undergoing development
because of its observed activity against these
kinases. | [General Description]
non-receptor tyrosine kinase|
Treatment: CML, ALL, GIST
Oral bioavailability = 98%
Elimination half-life = 20 h
Protein binding = 95% | [Biological Activity]
imatinib is an inhibitor of protein-tyrosine kinase with ic50 values of 0.1, 0.1 and 0.025μm, respectively against pdgf receptor, c-kit and abl [1].the type 3 group of receptor tyrosine kinases includes pdgfr, csf-1r, flt-3, c-kit and so on. pdgfrs are found in normal tissues, cells as well as some tumors. it is associated with several nonmalignant proliferative diseases. in vitro assays show that imatinib can inhibit both pdgf-aa and pdgf-bb stimulated pdgf receptor phosphorylation. imatinib is also found to inhibit the phosphorylation of c-kit, another kinase which mediates the growth of a number of tumors. imatinib inhibits the phosphorylation of these kinases without effecting the expression of them. some other kinases of the type 3 group (such as fms and flt-3) can’t be inhibited by imatinib, suggesting a selectivity of imatinib. in addition, imatinib is shown to significantly inhibit the bcr-abl tyrosine kinase both in cell-based assay and in vitro kinase assay. moreover, as a downstream pathway of pdgf-mediated signals, map kinase activation can also be effected in rat a10 smooth muscle cells [1]. | [Clinical Use]
Tyrosine kinase inhibitor, antineoplastic agent:
Treatment of chronic myeloid leukaemia
Treatment of metastatic malignant gastrointestinal
stromal tumours
Treatment of acute lymphoblastic leukaemia | [target]
PDGF receptor | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin
- avoid.
Anticoagulants: enhanced anticoagulant effect of
warfarin, replace with heparin.
Antidepressants: concentration reduced by St. Johns
Wort - avoid.
Antiepileptics: concentration reduced by
carbamazepine, fosphenytoin, oxcarbazepine and
phenytoin - avoid; absorption of phenytoin possibly
reduced.
Antipsychotics: avoid concomitant use with
clozapine (increased risk of agranulocytosis).
Antivirals: avoid with boceprevir.
Ciclosporin: may increase ciclosporin levels.
Cytotoxics: possibly increases bosutinib
concentration - avoid or reduce bosutinib dose;
concentration of everolimus and possibly ibrutinib
increased - reduce dose of everolimus and ibrutinib.
Tacrolimus: may increase tacrolimus levels. | [Metabolism]
The main circulating metabolite in humans is the
N-demethylated piperazine derivative, which shows
similar in vitro potency to the parent. Imatinib and the
N-demethyl metabolite together accounted for about
65% of the circulating radioactivity (AUC(0-48h)). The
remaining circulating radioactivity consisted of a number
of minor metabolites. In vitro results showed that
CYP3A4 was the major human P450 enzyme catalysing
the biotransformation of imatinib. Based on the recovery
of compound(s) after an oral [14C]-labelled dose of
imatinib, approximately 81% of the dose was recovered
within 7 days in faeces (68% of dose) and urine (13% of
dose). Unchanged imatinib accounted for 25% of the dose
(5% urine, 20% faeces), the remainder being metabolites. | [Mode of action]
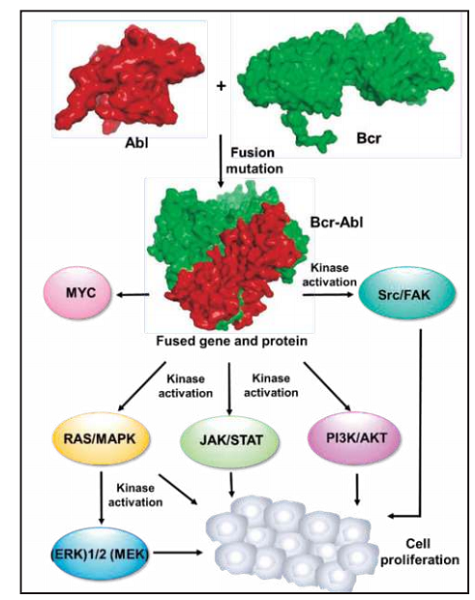
Imatinib binds close to the ATP-binding site of BCR-ABL and locks it in a closed inactive conformation which excludes ATP. This shuts down its sustained signaling, thus blocking leukemic cell growth.
| [References]
[1] elisabeth buchdunger, catherine l. cioffi, norman law, david stover, sayuri ohno-jones, brian j. druker and nicholas b. lydon. abl protein-tyrosine kinase inhibitor sti571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. the journal of pharmacology and experimental therapeutics. 2000, 295(1): 139-145. |
| Questions And Answer | Back Directory | [Product description]
Imatinib is a kind of oral drugs used for the treatment of the chronic myelogenous leukemia (abbreviated CML) of positive symptoms of Philadelphia chromosome (Ber-Abl). It is suitable for being applied to adult patients in acute transformation phase, accelerated phase, and chronic phase with treatment failure with interferon.
CML is a kind of hematopoietic stem cell disease caused by the DNA abnormalities in the bone marrow stem cells. DNA abnormalities will produce abnormal proteins and interfere with the normal generation process of the white blood cells in the bone marrow, resulting in the sharp increase in the number of white blood cells. CML is divided into three phases including chronic phase, accelerated phase and crisis phase with the average survival period of the patients in crisis phase being only 2-3 months.
Imatinib is also effective in treating the gastrointestinal stromal tumor with the efficiency being about 50%.
| [Binding Mode]
Imatinib binds with high specificity to an inactive form of the ABL kinase, resulting in high kinase selectivity. Interestingly, the N-methylpiperazine group, which was installed to increase solubility, also interacts strongly with ABL via hydrogen bonds to the backbone carbonyl group of Ile360 and His361. The co-crystal structure of imatinib with ABL shows additional four hydrogen bonds between (1) the pyridine ring N atom and the backbone NH of Met318 in the hinge region; (2) the anilino NH and the side chain of the gatekeeper ;residue Thr315; (3) the amide NH and the side chain of Glu286 from helix C; and (4) the amide carbonyl and the backbone NH of Asp381 (Fig. 4). The hydrogen bonds are also complemented by extensive hydrophobic interactions over the whole length of the inhibitor except the N-methylpiperazine region which is partially exposed to solvent. | [Approved indications in Europe, the United States and other countries]
Novartis's imatinib (imatinib, Glivec) had been approved in Switzerland for being used as first-line drug for the treatment of early-stage adult chronic myelogenous leukemia and can also be applied to patients with various types of chronic granulocytic leukemia. Switzerland is the first countries which had approved to additionally include the above two indications of this drug.
On July 28, 2006, the European Agency for the Evaluation of Medicinal Products (EMEA) had recommended the imatinib (Gleevec) of the Novartis Company for the treatment of two new indications-dermatofibrosarcoma protuberans (DFSP) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL). These final approvals of these two indications still need to wait the decision of the European Food and Drug Administration.
In addition, Novartis has announced that the application of imatinib in treating hypereosinophilic syndrome and systemic mastocytosis is still in the approval process of FDA and EMEA.
The drug has currently been approved in Europe, the United States and other countries for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia (Ph + CML) and gastrointestinal stromal tumors.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| [Precautions]
When combined with Ketoconazole, itraconazole, erythromycin, clarithromycin, the Cmax of this drug can (the maximum plasma concentration after the first drug administration) be increased by an average of 26% with the AUC being increased by 40%.
The inducers of the metabolizing enzyme of hepatic drugs such as dexamethasone, phenytoin, carbamazepine, rifampicin and barbiturates can significantly reduce blood concentration of the drug.
When the CYP3A4 metabolized substrates such as simvastatin, cyclosporine, pimozide, etc. were used in combined with imatinib, the plasma concentration of those drugs can be increased due to its competing with the drug enzyme.
| [Uses]
Imatinib can be used for the treatment of the chronic myelogenous leukemia (abbreviated CML) of positive symptoms of Philadelphia chromosome (Ber-Abl). It is suitable for being applied to adult patients in acute transformation phase, accelerated phase, and chronic phase with treatment failure with interferon.
|
|
|