| Identification | More | [Name]
Melphalan | [CAS]
148-82-3 | [Synonyms]
4-[BIS(2-CHLOROETHYL)AMINO]-L-PHENYLALANINE
L-PAM
L-PHENYL-ALANINE MUSTARD
PHENYLALANINE MUSTARD
3-(p-(bis(2-chloroethyl)amino)phenyl)-l-alanine
3(p-(bis(2-chloroethyl)amino)phenyl)-l-alanine
3025c.b.
3025cb
3-p-(di(2-chloroethyl)amino)-phenyl-l-alanine
4-(bis(2-chloroethyl)amino)-l-phenylalanin
alaninenitrogenmustard
at-290
cb3025
l-3-(p-(bis(2-chloroethyl)amino)phenyl)alanine
l-3-(para-(bis(2-chloroethyl)amino)phenyl)alanine
levofalan
l-sarcolysin
l-sarcolysine
l-sarkolysin
melfalan | [EINECS(EC#)]
205-726-3 | [Molecular Formula]
C13H18Cl2N2O2 | [MDL Number]
MFCD00057717 | [Molecular Weight]
305.2 | [MOL File]
148-82-3.mol |
| Chemical Properties | Back Directory | [Appearance]
Melphalan forms solvated crystals from methanol. | [Melting point ]
~180 °C | [alpha ]
D25 +7.5° (c = 1.33 in 1.0N HCl); D22 -31.5° (c = 0.67 in methanol) | [Boiling point ]
473.1±45.0 °C(Predicted) | [density ]
1.3587 (rough estimate) | [refractive index ]
1.6070 (estimate) | [storage temp. ]
-20°C Freezer | [solubility ]
95% ethanol and 1 drop 6 N HCl: 0.05 g/mL, clear
| [form ]
powder
| [pka]
pKa 1.42/2.75/9.17(H2O,t =37.0,I=0.5) (Uncertain) | [color ]
white
| [Water Solubility ]
<0.1 g/100 mL at 22 ºC | [Usage]
Antineoplastic | [Merck ]
13,5850 | [BRN ]
2816456 | [InChIKey]
SGDBTWWWUNNDEQ-LBPRGKRZSA-N | [CAS DataBase Reference]
148-82-3(CAS DataBase Reference) | [IARC]
1 (Vol. 9, Sup 7, 100A) 2012 | [EPA Substance Registry System]
148-82-3(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T+ | [Risk Statements ]
R45:May cause cancer.
R26/27/28:Very Toxic by inhalation, in contact with skin and if swallowed .
R63:Possible risk of harm to the unborn child. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S22:Do not breathe dust .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2811 6.1/PG 2
| [WGK Germany ]
3
| [RTECS ]
AY3675000
| [F ]
8 | [HazardClass ]
6.1(a) | [PackingGroup ]
II | [HS Code ]
29224999 | [Safety Profile]
Confirmed human carcinogen producing leukemia and Hodgkin's disease. Poison by ingestion, intravenous, and intracerebral routes. Human systemic effects by ingestion: nausea, hypermothty, diarrhea, agranulocytosis, thrombocytopenia. Human reproductive effects by ingestion: menstrual changes. Mutation data reported. A skin irritant. Used as a poison gas. When heated to decomposition it emits toxic fumes of ClandNOx. | [Hazardous Substances Data]
148-82-3(Hazardous Substances Data) | [Toxicity]
LD50 i.p. in rats: 14.7 mmol/kg (Ross) |
| Hazard Information | Back Directory | [General Description]
White to buff-colored powder. Odorless or with a faint odor. An antineoplastic medicine. | [Reactivity Profile]
MELPHALAN(148-82-3) is a nitrogen mustard. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Strong irritant to eyes and mucous membranes. Confirmed carcinogen. | [Potential Exposure]
An alkylating agent. Healthcare workers may be exposed. As a drug it is an immunosuppressant,
used in the treatment of multiple myeloma and cancer of
the ovary. It is also used in investigation of other types
of cancer and as an antineoplastic in animals. Human
exposure to melphalan occurs principally during its use in
cancer treatment. Melphalan is administered orally or intravenously. Adult dosage is 6 mg/day, 5 days per month. Has
been used as a military poison gas (a nitrogen mustard,
alkaline, crystals). | [Fire Hazard]
Flash point data are not available for this chemical; however MELPHALAN is probably combustible. | [First aid]
Move victim to fresh air. Call 911 or emergency
medical service. Give artificial respiration if victim is not
breathing. Do not use mouth-to-mouth method if victim
ingested or inhaled the substance; give artificial respiration with the aid of a pocket mask equipped with a one-way
valve or other proper respiratory medical device.
Administer oxygen if breathing is difficult. Remove and
isolate contaminated clothing and shoes. Keep victim warm
and quiet. Effects of exposure (inhalation, ingestion, or
skin contact) to substance may be delayed. Ensure that
medical personnel are aware of the material(s) involved
and take precautions to protect themselves. Medical observation is recommended for 24 to 48 hours after breathing
overexposure, as pulmonary edema may be delayed.
As first aid for pulmonary edema, a doctor or authorized
paramedic may consider administering a drug or other inhalation therapy. Skin Contact: Flood all areas of body
that have contacted the substance with water. Speed in
removing material from skin is of extreme importance.
Don’t wait to remove contaminated clothing; do it under
the water stream. Use soap to help assure removal. Isolate
contaminated clothing when removed to prevent contact by
others. Eye Contact: Remove any contact lenses at once.
Immediately flush eyes well with copious quantities
of water or normal saline for at least 20 to 30 minutes.
Seek medical attention. Inhalation: Leave contaminated
area immediately; move to the fresh air. Proper respiratory
protection must be supplied to any rescuers. If coughing,
difficult breathing or any other symptoms develop, seek
medical attention at once, even if symptoms develop many
hours after exposure. Ingestion: Contact a physician, hospital, or poison center at once. If the victim is unconscious or
convulsing, do not induce vomiting or give anything by
mouth. Assure the his airway is open and lay him on his
side with his head lower than his body and transport immediately to a medical facility. If conscious and not convulsing, give a glass of water to dilute the substance. Vomiting
should not be induced without a physician’s advice | [Shipping]
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. UN3249 Medicine, solid, toxic, n.o.s.,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials | [Description]
Melphalan is a nitrogen mustard derivative of the large neutral
amino acid L-phenylalanine. It was first synthesized in 1953 by
Bergel and Stock and is the active L-isomer of the compound.
The D-isomer, known as medphalan, is less active against
certain animal tumors, and the dose needed to produce effects
on chromosomes is larger than that required with the L-isomer.
The racemic (DL-) form is known as merphalan or sarcolysin. | [Description]
Melphalan is a phenylalanine derivative of mechlorethamine, belonging to the family of nitrogen mustard alkylating agents that are used for chemotherapy.1 It attaches an alkyl group to guanine bases in DNA at the 7-nitrogen of the imidazole ring, which leads to DNA interstrand and intrastrand crosslinks, cytotoxicity, and apoptosis.1 Melphalan can inhibit the growth of PC-3 prostate cancer cells with IC50 values of 0.074 or 0.77 μM for sequential dosing or single dosing, respectively.2 | [Chemical Properties]
Crystalline Solid | [Chemical Properties]
Melphalan forms solvated crystals from methanol. | [Waste Disposal]
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform to EPA regulations governing
storage, transportation, treatment, and waste disposal. It is
inappropriate and possibly dangerous to the environment to
dispose of expired or waste drugs and pharmaceuticals by
flushing them down the toilet or discarding them to the
trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds,
double-bagged in plastic, discard in trash. Larger quantities
shall carefully take into consideration applicable DEA,
EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being
careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be
labeled, securely packaged, and transported by a state
licensed medical waste contractor to dispose by burial
in a licensed hazardous or toxic waste landfill or
incinerat | [Originator]
Alkeran,Burroughs-Wellcome,US,1964 | [Uses]
antineoplastic, alkylating agent | [Uses]
Glucocorticoid | [Uses]
Melphalan USP (Alkeran) is used to treat multiple myeloma; plasmacytic myeloma; cancer of breast and ovary. | [Definition]
ChEBI: A phenylalanine derivative comprising L-phenylalanine having [bis(2-chloroethyl)amino group at the 4-position on the phenyl ring. | [Indications]
Melphalan (Alkeran) is an amino acid derivative of
mechlorethamine that possesses the same general spectrum
of antitumor activity as do the other nitrogen mustards.
However, the bioavailability of the oral preparation
is quite variable (25–90%) from one patient to
another.
The major indications for melphalan are in the palliative
therapy of multiple myeloma and cancers of the
breast or ovary. Because it does not produce alopecia,
melphalan is occasionally substituted for cyclophosphamide
in the CMF regimen for breast cancer.
Melphalan produces less nausea and vomiting than
does cyclophosphamide; however, its bone marrow suppression
tends to be more prolonged and affects both
white cells and platelets. Peak suppression of blood
counts occurs 14 to 21 days after a 5-day course of drug
therapy; recovery is generally complete within 3 to 5
weeks. | [Manufacturing Process]
Diethyl sodium phthalimidomalonate (Barger and Weichselbaum, Organic
Syntheses, 1943, Coll. Vol. II, 384) (6.52 g) was dissolved in boiling methyl
ethyl ketone (80 ml) and a solution of p-nitrobenzyl chloride (3.44 g; 1.0 mol)
in the same solvent (20 ml) was added, Sodium iodide (ca 0.5 g) dissolved in
hot methyl ethyl ketone (10 ml) was introduced, and produced an immediate
precipitation. The mixture was refluxed for 1.5 hours, cooled, filtered,
evaporated under vacuum and the residual gum crystallized from ethanol. The
di-ethyl-p-nitrobenzyl-phthalimidomalonate formed colorless prisms (88%),
MP 103° to 105°C, sharpening to 104° to 105°C on recrystallizing from
ethanol.
Diethyl-p-nitrobenzyl-phthalimidomalonate (70 g) and sodium carbonate (70
g) in water (700 ml) were refluxed overnight with mechanical stirring (to
avoid bumping). The clear brown solution was acidified with hydrochloric acid
and refluxing and stirring were continued for a further 40 minutes. The
mixture was cooled and the colorless precipitate (31 g) collected. A second
crop (18.5 g) was obtained on evaporation of the mother liquors.
Crystallization from aqueous ethanol gave the compound N-carboxybenzoyl-p-nitro-DL-phenylalanine as small needles, MP 198° to 200°C.
The N-carboxybenzoyl compound (2.7 g) was refluxed for 30 minutes with
acetic anhydride (10 ml), the mixture taken to dryness (vacuum) and the
residue heated with water. The cooled gummy product became granular on
rubbing and crystallized from methyl ethyl ketone-petrol or aqueous ethanol in
almost colorless needles, MP 184° to 186°C, of p-nitro-N-phthaloyl-DLphenylalanine.
A solution of p-nitro-N-phthaloyl-DL-phenylalanine (1.0 g) in methanol (25 ml)
and a solution of cinchonidine (0.865 g) in methanol (30 ml) were mixed.
Crystallization soon set in. The mixture was left overnight, and the colorless
needles (0.97 g), MP 209° to 210°C, collected. After two recrystallizations
from methanol the cinchonidine salt of the D-acid had MP 211°C.
Evaporation of the mother liquors from the original cinchonidine experiment
gave a gum which crystallized readily from aqueous ethanol in almost
colorless needles (0.73 g), MP 191° to 192.5°C. Two recrystallizations from
aqueous ethanol gave the cinchonidine salt of the L-acid, MP 192.5° to 194°C.
To the salt (2.9 g) in warm ethanol (50 ml) was added water (50 ml) and a
slight excess (ca 10 ml) of N aqueous sodium hydroxide. The mixture was
diluted with water, cooled, filtered from the precipitated base and the filtrate
acidified with hydrochloric acid. Refluxing with 2 N ethanolic hydrogen chloride
yielded p-nitro-N-phthaloyl-L-phenylalanine ethyl ester, according to US Patent
3,032,585.
Then, as described in US Patent 3,032,584, ethyl N-phthaloyl pnitrophenylalaninate (9.0 g) was hydrogenated in a mixture of ethyl acetate
(120 g) and methanol (80 g) with a palladium-calcium carbonate (1% Pd)
catalyst (1.4 g). When gas uptake was complete, the filtrate from the
hydrogenation mixture was evaporated under reduced pressure. The residual
gum was taken up in ether, the solution filtered, and a slight excess of a dry
ethereal hydrogen chloride solution added slowly with stirring. The gummy
precipitate became granular on rubbing and the ether-washed product was
crystallized from ethyl acetate-acetone [1st crop, 2.8 g, MP 188° to 192°C
(decomp.); 2nd crop, 3.9 g, MP 189° to 192°C (decomp.)] . Part of the first
batch was recrystallized from ethyl acetate and gave very slightly tinted
needles, MP 188° to 190°C (decomp.) of ethyl N-phthaloyl paminophenylalaninate hydrochloride.
The free base was obtained from the hydrochloride by adding a slight excess
of dilute ammonium hydroxide to the aqueous solution, and crystallizing the
product from aqueous methanol. A further recrystallization with charcoal
treatment gave almost colorless needles, MP 110° to 112°C of ethyl Nphthaloyl p-aminophenylalaninate.
Ethyl N-phthaloyl p-aminophenylalaninate (3.15 g) (unrecrystallized) was
suspended in water (50 g) and glacial acetic acid (30 g) added. To the clear
solution, ethylene oxide (8.0 g) was added, the mixture allowed to stand for
17 hours, and then poured into water (350 g). The solution was neutralized
with sodium hydrogen carbonate and the liberated gum extracted with ether.
The ethereal solution was dried (magnesium sulfate) and evaporated. The
residual gum (3.95 g) was dissolved in benzene (50 g) and the solution dried
azeotropically by distilling off some of the solvent. Freshly distilled phosphorus oxychloride (8 g) was added and the mixture heated under reflux for 30
minutes.
The solvent was evaporated off under reduced pressure, and the residual gum
refluxed with concentrated hydrochloric acid (50 g) for 6 hours. The solution
was allowed to cool overnight. It was filtered from the phthalic acid crystals,
and freeze-dried, and to the pink residue was added acetone (160 g) and
ethyl acetate (50 g). The mixture was left in the cold room overnight and the
clear pink supernatant liquid poured off. The pink gummy hydrochloride
remaining in the flask was dissolved in water (20 g), saturated sodium acetate
solution added until precipitation was complete, and the product collected and
dried in a desiccator. The crude p-bis-(2-chloroethyl)-aminophenylalanine (3.6
g) was crystallized from methanol giving colorless needles, MP 172° to 174°C
(decomp.) of p-bis-(2-chloroethyl)-aminophenylalanine. | [Brand name]
Alkeran
(GlaxoSmithKline). | [Therapeutic Function]
Cancer chemotherapy | [Mechanism of action]
Melphalan is orally active, but absorption can be erratic. Absorption is decreased with food, but dosing regimens do not demand an empty stomach. The drug can be formulated for IV administration, but the risk of serious side effects is higher. Melphalan distributes into body water, so toxicity can be pronounced in dehydrated patients or in those with renal dysfunction. Dehydration can be corrected, but dosage adjustments should be considered in patients with renal disease. | [Clinical Use]
This aromatic mustard, used primarily in the treatment of multiple myeloma, is able to stabilize the lone pair of electrons on the mustard nitrogen through resonance with the conjugated phenyl ring, slowing the formation of the reactive aziridinium ion. | [Side effects]
Because the lone pair of electrons of melphalan (and other aromatic mustards) is less reactive, there is a greater opportunity for distribution to cancer cells and a decreased incidence of severe side effects. There is a lower incidence of nausea and vomiting compared to mechlorethamine, but patients still experience myelosuppression, which can be severe. This drug also is mutagenic and can induce leukemia. | [Synthesis]
Melphalan, L-3-[p-[bis-(2-chloroethyl)amino]phenyl]alanine (30.2.1.13), is a
structural analog of chlorambucil in which the butyric acid fragment is replaced with an
aminoacid fragment, alanine. This drug is synthesized from L-phenylalanine, the nitration of which with nitric acid gives 4-nitro-L-phenylalanine (30.2.1.8). Reacting this with an ethanol
in the presence of hydrogen chloride gives the hydrochloride of 4-nitro-L-phenylalanine ethyl
ester (30.2.1.9), the amino group of which is protected by changing it to phthalamide by a
reaction with succinic anhydride to give 30.2.1.10. The nitro group in this molecule is
reduced to an amino group using palladium on calcium carbonate as a catalyst. The result�ing aromatic amine (30.2.1.11) is then reacted with ethylene oxide, which forms a bis-(2-
hydroxyethyl)-amino derivative (30.2.1.12). The hydroxy groups in this molecule are
replaced with chlorine atoms upon reaction with thionyl chloride, after which treatment with
hydrochloric acid removes the phthalamide protection, giving melphalan (30.2.13).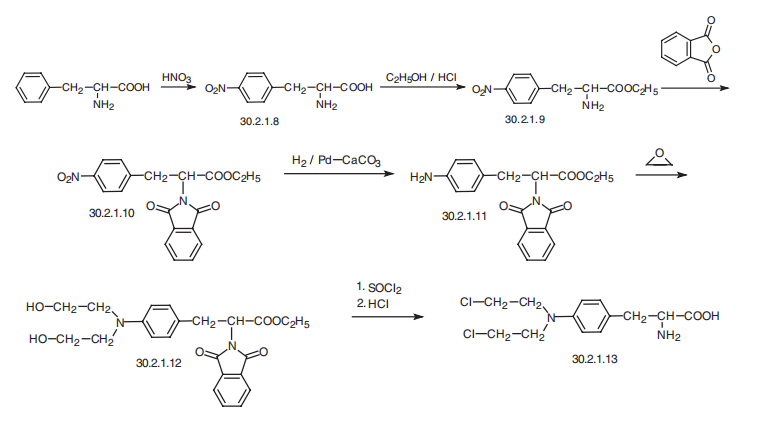
| [Carcinogenicity]
Melphalan is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans. | [Environmental Fate]
The release to the environment of melphalan may result
through various waste streams. It is practically insoluble in
water, insoluble in chloroform and ether, slightly soluble in
methanol, and soluble in ethanol, propylene glycol, 2% carboxymethyl
cellulose, and alkaline and dilute acid solutions. It
hydrolyzes in aqueous solution.
If released into water, melphalan is expected to adsorb to
suspended solids and sediment in the water based on the
estimated Koc, using a structure estimation method based on
molecular connectivity indices, of 355. Volatilization from
water surfaces is not expected to be an important fate process
based on this compound’s estimated Henry’s law constant,
developed using a fragment constant estimation method, of
4.2×10-13 atmm3 mol-1 and its estimated vapor pressure,
according to a model of gas/particle partitioning of semivolatile
organic compounds in the atmosphere of 3×10-10mmHg at
25 �C. Hydrolysis in water and in moist soil will be an important
fate process according to melphalan’s experimental neutral
aqueous hydrolysis rate constant at 25℃ of 0.15 h-1 which
corresponds to a half-life of 4.6 h at pH 7. In line with soil
compartment, insufficient data are available to determine the
rate or importance of biodegradation of melphalan in water.
If released to soil, it is expected to have moderate mobility
based on its estimated Koc. Volatilization from moist soil
surfaces is not expected to be an important fate process based
on its estimated Henry’s law constant, or from dry soil surfaces,
based on its estimated vapor pressure. Contrarily, hydrolysis in
moist soil may be an important fate process according to its
experimental neutral aqueous hydrolysis rate constant and its
half-life. There are no available data to determine the rate or
importance of biodegradation of melphalan in soil.
If released to air, the value of its vapor pressure indicates
that it will exist solely in the particulate phase in the atmosphere.
Melphalan will be removed from the atmosphere by
wet or dry deposition. On other hand, vapor-phase melphalan
will be degraded in the atmosphere by reaction with photochemically
produced hydroxyl radicals with an estimated halflife
of about 1.7 h.
An estimated bioconcentration factor value of 0.24, from an
experimental log Kow of -0.52, suggests that the potential for
bioconcentration in aquatic organisms is very low. | [Metabolism]
Spontaneous hydrolysis degradation rather than
enzymatic metabolism. Percentage of dose excreted in
the urine as active or toxic moiety ranges from 11-93%;
20-50% excreted in the faeces within 6 days | [storage]
Store at RT | [Purification Methods]
Purify melphalan by recrystallisation from MeOH, and its solubility is 5% in 95% EtOH containing one drop of 6N HCl. It is soluble in EtOH and propylene glycol but is almost insoluble in H2O. The RS-form has m 180-181o, and the R-form crystallises from MeOH with m 181.5-182o and [�] D21 -7.5o (c 1.26, 1.0 N HCl). [Bergel & Stock J Chem Soc 2409 1954, Beilstein 14 IV 1689.] | [Toxicity evaluation]
Melphalan is a bifunctional alkylating agent of the nitrogen
mustard type that binds to cellular macromolecules and it is cell
cycle phase-nonspecific. This drug has the capacity to interfere
with normal mitosis and cell division in rapidly proliferating
tissues. Activity occurs as a result of formation of an unstable
ethylenimmonium ion, which alkylates or binds with many
intracellular molecular structures including nucleic acids. Its
cytotoxic action is primarily due to cross-linking of strands of
DNA and RNA, as well as inhibition of protein synthesis. |
|