| Identification | More | [Name]
3-Methyl-2-[pentanoyl-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]amino]-butanoic acid | [CAS]
137862-53-4 | [Synonyms]
3-methyl-2-[pentanoyl-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]amino]-butanoic acid
CGP-48933
DIOVAN
N-(1-OXOPENTYL)-N-[[2'-(1H-TETRAZOL-5-YL)[1,1'-BIPHENYL]-4-YL]METHYL]-L-VALINE
VALSARTAN
VALSARTAN, MM STANDARD
VALSARTAN, USP STANDARD
VALSARTAN, RELATED COMPOUND A(R)-N-VALERYL-N-([2'-(1-H-TETRAZOLE)-5-YL)-BIPHENYL-4-YL]-METHYL)-VALINE USP STANDARD
VALSARTAN, RELATED COMPOUND C(S)-N-VALERYL-N-([2'-(1-H-TETRAZOLE)-5-YL)-BIPHENYL-4-YL]-METHYL)-VALINE BENZYL ESTER USP STANDARD
Valsartan99%
N-[[(2-Cyano(1.1-Biphenyl)-4-Yl)Methyl]MethylEster]-L-Valine.Hydrochloride
ValsartanC24H29N503
Valsartane
Diovan, N-(1-Oxopentyl)-N-[[2(1H-tetrazol-5-yl)[1,1biphenyl]-4-yl]methyl]-L-valine, CGP-48933
N-(1-N.PENTANOYL)-N-[[2''-(1H-TETRAZOL-5-YL)[1'',1-BIPHENYL]-4-YL]METHYL]-L-VALINE/VALSARTAN
L-Valine, N-(1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-(9CI)
Nisis
Tareg
Valsratan
(S)-2-(N-((2''-(1H-TETRAZOL-5-YL)BIPHENYL-4-YL)METHYL)PENTANAMIDO)-3-METHYLBUTANOIC ACID | [EINECS(EC#)]
1592732-453-0 | [Molecular Formula]
C24H29N5O3 | [MDL Number]
MFCD00865840 | [Molecular Weight]
435.52 | [MOL File]
137862-53-4.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Powder | [Melting point ]
116-117°C | [Boiling point ]
684.9±65.0 °C(Predicted) | [density ]
1.212±0.06 g/cm3(Predicted) | [vapor pressure ]
0-0Pa at 20-25℃ | [storage temp. ]
2-8°C | [solubility ]
DMSO: ≥20mg/mL | [form ]
powder | [pka]
3.56±0.10(Predicted) | [color ]
white to tan | [optical activity]
[α]/D -55 to -70°, c = 1 in methanol | [Water Solubility ]
84.99mg/L(25 ºC) | [Usage]
A nonpeptide angiotensin II AT1-receptor antagonist | [Merck ]
14,9916 | [BCS Class]
2 | [Stability:]
Hygroscopic | [InChIKey]
ACWBQPMHZXGDFX-QFIPXVFZSA-N | [LogP]
1.2-2.8 at 25℃ and pH2.5-7 | [Surface tension]
59.6-68.1mN/m at 10-100mg/L and 20℃ | [Dissociation constant]
3.76-4.38 at 25℃ | [CAS DataBase Reference]
137862-53-4(CAS DataBase Reference) | [EPA Substance Registry System]
L-Valine, N-(1-oxopentyl)-N-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]- (137862-53-4) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3 | [RTECS ]
YV9455000 | [HS Code ]
29339900 | [Hazardous Substances Data]
137862-53-4(Hazardous Substances Data) |
| Questions And Answer | Back Directory | [Indications and Usage]
Valsartan (137862-53-4) is a type of specific angiotensin I (AT1) receptor antagonist and a clinical non-dermal AT1 receptor antagonist following losartan that has important effects in adjusting bodily blood pressure and maintaining electrolyte-body fluid balance. Valsartan’s antihypertensive effects are stronger than those of enalapril and is suitable for treating hypertension, mild to moderate primary hypertension, and especially secondary hypertension caused by renal damage. It can significantly reduce proteinuria for hypertension patients with diabetes or normal liver functions, and it can promote uric acid and urinary sodium to protect the kidney. Valsartan is also suitable for reducing the cardiovascular mortality for high risk patients (left ventricular failure or dysfunction) after experiencing a heart attack.
| [Mechanisms of Action]
Valsartan (137862-53-4) selectively affects the AT1 receptor subtype and prevents AT1 from binding with AT1 receptors (its selective AT1 receptor antagonizing effect is about 20000 times greater than its effects on AT2 receptors), thus inhibiting vasoconstriction and aldosterone release, producing an antihypertensive effect, but it cannot inhibit the release of aldosterone caused by potassium ions (K+). Valsartan does not affect angiotensin-converting enzymes (ACE) or renin and its receptors, and it does not inhibit ion channels related to blood pressure regulation and sodium balance. Valsartan does not inhibit ACE and does not affect bodily bradykinin levels, thus causing less coughing side effects than ACE inhibitors. Valsartan does not affect heart rate when lowering blood pressure. Sudden ceasing in Valsartan use will not cause rebound hypertension or other side effects. Valsartan does not affect hypertension patients’ overall cholesterol, triglyceride, blood glucose, or uric acid levels.
| [Pharmacokinetics]
For most patients, a single oral dose will have antihypertensive effects that occur within 2 hours, peak at 4-6 hours, and continue for over 24 hours. 2-4 weeks of treatment will result in maximum hypertensive curative effects, which will last throughout long-term treatment. It can be used with thiazide diuretics to further strengthen antihypertensive effects.
| [Clinical Research]
A clinical trial showed that Valsartan has very noticeable effects on mild to moderate hypertension. A daily dose of ≥80 mg can effectively control systolic and diastolic blood pressure while not affecting blood pressure’s circadian rhythm; a daily 160mg dose has more noticeable effects than a daily 100mg dose of losartan. Moderate hypertension patients with intact renal functions have a good tolerance towards Valsartan, Valsartan’s curative effects are significantly superior to those of ACE inhibitors, and there are minimal adverse reactions. Effective in treating severe hypertension when combined with other antihypertensive drugs.
|
| Hazard Information | Back Directory | [Description]
Diovan was launched in Germany and the UK as an angiotensin Ⅱ
antagonist for use as an antihypertensive agent. Biphenylbromomethyl nitrite serves
as the starting material for a three step synthesis of the compound, in which the (S)-
enantiomer is more active than the (R)-enantiomer. Valsartan is a nonpeptide drug
which is a highly specific antagonist of the AT1 receptor and is potent and orally
active. This receptor is responsible for angiotensin Ⅱ cardiovascular effects
(aldosterone and catecholamine secretion, vascular constriction, positive inotropic
response and renal effects). Unlike losartan, it is not a prodrug and a single daily
dose is comparible in activity to the ACE drug enalapril. It also did not exhibit the
coughing side effect observed with ACE inhibitors. Diovan is slowly metabolized
(long lasting) with its main metabolite being significantly less active. There was no
evidence of rebound hypertension when drug treatment was terminated and was as
effective as the dihydropyridine Ca antagonist anlodipine. | [Chemical Properties]
White Crystalline Powder | [Originator]
Norvartis (Switzerland) | [Uses]
A nonpeptide angiotensin II AT1-receptor antagonist. Antihypertensive. | [Uses]
Angiotensin II inhibitor, antihypertensive | [Uses]
May be used as a first line agent to treat uncomplicated hypertension, isolated systolic hypertension and left ventricular hypertrophy. May be used as a first line agent to delay progression of diabetic nephropathy. Losartan may be also used as a second l | [Definition]
ChEBI: A monocarboxylic acid amide consisting of L-valine in which the amino hydrogens have been replaced by a pentanoyl and a [2'-(1H-tetrazol-5-yl)biphenyl]-4-yl]methyl group. It exhibits antihypertensive activity. | [Manufacturing Process]
0.5 g of 2'-cyanobiphenyl-4-carbaldehyde, 2.5 g of molecular sieve 5 A in
tetrahydrofuran with stirring at room temperature for 36 hours; then the
reaction mixture was cooled to 0°-5°C, 0.815 g of (L)-valine methyl ester
hydrochloride and 180 mg of sodium cyanoborohydride dissolved in 4.8 ml of
methanol are added. The mixture is stirred at room temperature for 24 hours
and then concentrated in vacuo yields N-[(2'-cyanobiphenyl-4-yl)methyl]-(L)-
valine methyl ester after flash chromatography.
1.15 g of N-[(2'-cyanobiphenyl-4-yl)methyl]-(L)-valine methyl ester, 0.625 ml
of triethylamine in 9 ml of dichloromethane are treated 0.56 ml of n-valeryl
chloride at 0°C and stirred at room temperature overnight and then
evaporated to dryness. The residue is taken up in diethyl ether and the diethyl
ether mixture is washed with sodium hydrogencarbonate solution and brine.
Flash chromatography (180 g of silica gel; ethyl acetate/petroleum ether 1:1)
yields N-valeryl-N-[(2'-cyanobiphenyl-4-yl)methyl]-(L)-valine methyl ester.
(S)-N-(1-Carboxy-2-methyl-prop-1-yl)-N-pentanoyl-N-[2'-(1H-tetrazol-5-
yl)biphenyl-4-ylmethyl]-amine. The product can be prepared starting from
1.40 g of N-valeryl-N-[(2'-cyanobiphenyl-4-yl)methyl]-(L)-valine methyl ester
and 2.25 g of tributyltin azide with subsequent flash chromatography; melting
interval 105°-115°C (from ethyl acetate). | [Brand name]
Diovan (Novartis). | [Therapeutic Function]
Antihypertensive | [General Description]
Valsartan (137862-53-4), N-(1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine (Diovan), likelosartan, possesses the acidic tetrazole system, which mostlikely plays a role, similar to that of the acidic groups of angiotensinII, in binding to the angiotensin II receptor. In addition,the biphenyl system that serves to separate the tetrazolefrom the aliphatic nitrogen is still present. In addition, there isa carboxylic acid side chain in the valine moiety that alsoserves to bind to the angiotensin II receptor.
| [Biochem/physiol Actions]
Valsartan is an Angiotensin II type 1 (AT1) receptor antagonist and anti-hypertensive. Valsartan renders protection against heart attack and stroke resulting from abrupt increase in blood pressure. Valsartan reduces myocardial-infarction-related complications in heart attack survivors. | [Clinical Use]
Angiotensin-II antagonist:
Hypertension
Heart failure
Myocardial infarction with left ventricular failure | [Synthesis]
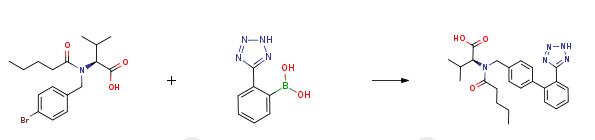
An aqueous solution (120 ml) of potassium hydroxide (0.568 mol, 31.8 g) is added in succession with 2-[(4-bromo-benzyl)-pentanoyl-amino]-3-methyl-butyric acid (0.811 mol, 30.0 g), tetrahydrofuran (120 ml), triphenylphosphine (0.0121 mol, 3.2 g) and palladium acetate (0.00405 mol, 0.91 g). The reaction mixture is refluxed and added with 2-(2H-tetrazol-5-yl)-benzene-boronic acid (0.142 mol, 27.0 g) in portions in about 6 h. After completion of the addition, the mixture is left to react for 2h, then cooled to room temperature and the phases are separated. The organic phase is diluted with water (120 ml) and tetrahydrofuran is distilled off under reduced pressure. The remaining aqueous solution is acidified to pH 6.5 and washed with isopropyl acetate (60 ml). The aqueous phase is acidified to pH 2 and diluted with isopropyl acetate (60 ml), the diphasic solution is filtered to remove phenyltetrazol. Phases are separated and the organic phase is concentrated under reduced pressure, to obtain a thick oil that is crystallized from isopropyl acetate (90 ml) and heptane (150 ml). The resulting product is filtered, washed twice with a 1:1 isopropyl acetate/heptane mixture (30 ml), and dried in static dryer at 45°C to obtain 28.2 g of Valsartan.
 | [Drug interactions]
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: antagonism of hypotensive effect and
increased risk of renal impairment with NSAIDs;
hyperkalaemia with ketorolac and other NSAIDs.
Antihypertensives: increased risk of hyperkalaemia,
hypotension and renal impairment with ACE-Is and
aliskiren.
Ciclosporin: increased risk of hyperkalaemia and
nephrotoxicity.
Diuretics: enhanced hypotensive effect;
hyperkalaemia with potassium-sparing diuretics.
ESAs: increased risk of hyperkalaemia; antagonism
of hypotensive effect.
Lithium: reduced excretion (possibility of enhanced
lithium toxicity).
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia and
nephrotoxicity. | [Metabolism]
Valsartan is not highly metabolised as only about 20
% of
dose is recovered as metabolites. A hydroxy metabolite
has been identified in plasma at low concentrations (less
than 10
% of the valsartan AUC). This metabolite is
pharmacologically inactive.
Valsartan is mainly eliminated by biliary excretion in
faeces (about 83
% of dose) and renally in urine (about
13
% of dose), mainly as unchanged drug. | [storage]
Store at RT | [References]
1) Criscione?et al. (1993),?Pharmacological profile of valsartan: a potent, orally active, nonpeptide antagonist of the angiotensin II AT1-receptor subtype; Br. J. Pharmacol.,?110?761
2) Wexler?et al. (1996), Nonpeptide angiotensin II receptor antagonists: the next generation in antihypertensive therapy; J. Med. Chem.,?39?625
3) Iwashita?et al. (2013),?Valsartan restores inflammatory response by macrophages in adipose and hepatic tissues of LPS-infused mice; Adipocyte,?2?28 |
|
|