- Triethylamine
-

- $300.00 / 150kg
-
2024-12-11
- CAS:121-44-8
- Min. Order: 1000kg
- Purity: 0.99
- Supply Ability: 3000 Metric Ton/Metric Tons per Year
- Triethylamine
-
- $0.00 / 200kg
-
2024-10-29
- CAS:121-44-8
- Min. Order: 20kg
- Purity: 99%
- Supply Ability: 20 tons
- Triethylamine
-

- $56.00 / 1kg
-
2024-10-25
- CAS:121-44-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 tons
Related articles - The toxic effects of Triethylamine
- Triethylamine is a colourless, unpleasant-smelling, inflammable liquid. It is absorbed well after both ingestion and inhalatio....
- Nov 5�����,2019
|
| | Triethylamine Chemical Properties |
| Melting point | -115 °C | | Boiling point | 90 °C | | density | 0.728 | | vapor density | 3.5 (vs air) | | vapor pressure | 51.75 mm Hg ( 20 °C) | | FEMA | 4246 | TRIETHYLAMINE | | refractive index | n20/D 1.401(lit.) | | Fp | 20 °F | | storage temp. | Store below +30°C. | | solubility | water: soluble112g/L at 20°C | | form | Liquid | | pka | 10.75(at 25℃) | | color | Clear | | Specific Gravity | 0.725 (20/4℃) | | Relative polarity | 1.8 | | PH | 12.7 (100g/l, H2O, 15℃)(IUCLID) | | Odor | Strong ammonia-like odor | | Odor Threshold | 0.0054ppm | | Odor Type | fishy | | explosive limit | 1.2-9.3%(V) | | Water Solubility | 133 g/L (20 ºC) | | JECFA Number | 1611 | | Merck | 14,9666 | | BRN | 1843166 | | Henry's Law Constant | 1.79 at 25 °C (Christie and Crisp, 1967) | | Exposure limits | NIOSH REL: IDLH 200 ppm; OSHA PEL: TWA 25 ppm (100 mg/m3); ACGIH
TLV: TWA 1 ppm, STEL 3 ppm (adopted). | | Dielectric constant | 5.0(Ambient) | | Stability: | Stable. Extremely flammable. Readily forms explosive mixtures with air. Note low flash point. Incompatible with strong oxidizing agents, strong acids, ketones, aldehydes, halogenated hydrocarbons. | | InChIKey | ZMANZCXQSJIPKH-UHFFFAOYSA-N | | LogP | 1.65 | | CAS DataBase Reference | 121-44-8(CAS DataBase Reference) | | NIST Chemistry Reference | Triethylamine(121-44-8) | | EPA Substance Registry System | Triethylamine (121-44-8) |
| | Triethylamine Usage And Synthesis |
| Outline | Triethylamine (formula: C6H15N), also known as N, N-diethylethanamine, is the most simple tri-substituted uniformly tertiary amine, having typical properties of tertiary amines, including salifying, oxidation, Hing Myers test (Hisberg reaction) for triethylamine does not respond. It is colorless to pale yellow transparent liquid, with a strong smell of ammonia, slightly fuming in the air. Boiling point: 89.5 ℃, relative density (water = 1): 0.70, the relative density (Air = 1): 3.48, slightly soluble in water, soluble in alcohol, ether. Aqueous solution is alkaline, flammable. Vapor and air can form explosive mixtures, the explosion limit is 1.2% to 8.0%. It is toxic, with a strong irritant.
| | Uses | Triethylamine is a clear, colorless liquid with an Ammonia or fish-like odor. It is used in making waterproofing agents, and as a catalyst, corrosion inhibitor and propellant.
It is mainly used as base, catalyst, solvent and raw material in organic synthesis and is generally abbreviated as Et3N, NEt3 or TEA. It can be used to prepare phosgene polycarbonate catalyst, polymerization inhibitor of tetrafluoroethylene, rubber vulcanization accelerator, special solvent in paint remover, enamel anti-hardener, surfactant, antiseptic, wetting agent, bactericides, ion exchange resins, dyes, fragrances, pharmaceuticals, high-energy fuels, and liquid rocket propellants, as a curing and hardening agent for polymers and for the desalination of seawater.
Consumption Quota of in medical industry:
Medicine
Consumption Quota(Unit: t/t)
Oxygen piperazine penicillin
0.584
Fluorine organism acid
10.00
Berberine hydrochloride
0.030
Adjacent benzene acetic acid
0.010
| | Production | It is produced by ethanol and ammonia in the presence of hydrogen, in containing Cu-Ni-clay catalyst reactor under heating conditions (190 ± 2 ℃ and 165 ± 2 ℃) reaction. The reaction also produces ethylamine and diethylamine, products were condensed and then absorption by ethanol spray to obtain crude triethylamine, through the final separation, dehydration and fractionation, pure triethylamine is obtained.
| | Reaction |
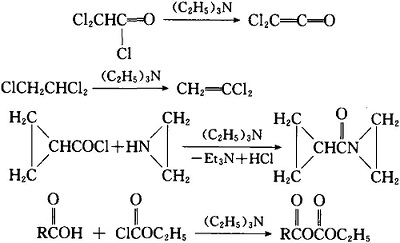
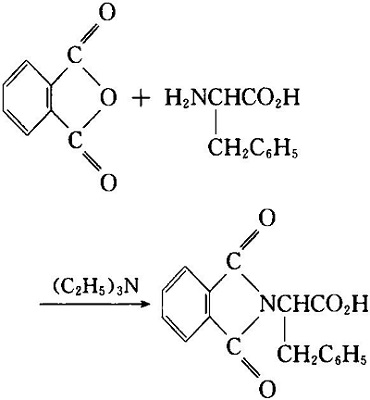
- It can be used to reduce the alkali in the reaction.
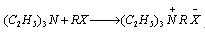
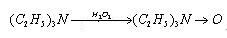
| | Health Effects | Triethylamine is a flammable liquid and a dangerous fire hazard. It can affect you when inhaled and by passing through the skin. Contact can severely irritate and bum the skin and eyes with possible eye damage. Exposure can irritate the eyes, nose and throat. Inhaling can irritate the lungs. Higher exposures may cause a build-up of fluid in the lungs (pulmonary edema), a medical mergency. It may cause a skin allergy and affect the liver and kidneys. | | Chemical Properties | Triethylamine is a colorless to yellowish liquid with a strong ammonia to fish-like odor. It is a base commonly used in organic chemistry to prepare esters and amides from acyl chlorides. Like other tertiary amines,it catalyzes the formation of urethane foams and epoxy resins. | | Physical properties | Clear, colorless to light yellow flammable liquid with a strong, penetrating, ammonia-like odor.
Experimentally determined detection and recognition odor threshold concentrations were <400
μg/m3 (<100 ppbv) and 1.1 mg/m3 (270 ppbv), respectively (Hellman and Small, 1974). An odor
threshold concentration of 0.032 ppbv was determined by a triangular odor bag method (Nagata
and Takeuchi, 1990). | | Uses | Triethylamine is a base used to prepare esters and amides from acyl chlorides as well as in the synthesis of quaternary ammonium compounds. It acts as a catalyst in the formation of urethane foams and epoxy resins, dehydrohalogeantion reactions, acid neutralizers for condensation reactions and Swern oxidations. It finds application in reverse phase high-performance liquid chromatography (HPLC) as a mobile-phase modifier. It is also used as an accelerator activator for rubber, as a propellant, as a corrosion inhibitor, as a curing and hardening agent for polymers and for the desalination of seawater. Furthermore, it is used in the automotive casting industry and the textile industry. | | Production Methods | Triethylamine is prepared by a vapor phase reaction of ammonia with ethanol or reaction of N,N-diethylacetamide with lithium aluminum hydride (Windholz et al 1983). It may also be produced from ethyl chloride and ammonia under heat and pressure (Hawley 1981) or by vapor phase alkylation of ammonia with ethanol (HSDB 1988). U.S. production is estimated at greater than 22,000 tons in 1972 (HSDB 1988). | | Definition | ChEBI: Triethylamine is a tertiary amine that is ammonia in which each hydrogen atom is substituted by an ethyl group. | | Application | Triethylamine (TEA, Et3N) is an aliphatic amine. It is used to catalytic solvent in chemical synthesis; accelerator activators for rubber; wetting, penetrating, and waterproofing agents of quaternary ammonium types; curing and hardening of polymers (e.g., corebinding resins); corrosion inhibitor; propellant.
Triethylamine has been used during the synthesis of:
5′-dimethoxytrityl-5-(fur-2-yl)-2′-deoxyuridine
3′-(2-cyanoethyl)diisopropylphosphoramidite-5′-dimethoxytrityl-5-(fur-2-yl)-2′-deoxyuridine
polyethylenimine600-β-cyclodextrin (PEI600-β-CyD)
It may be used as a homogeneous catalyst for the preparation of glycerol dicarbonate, via transesterification reaction between glycerol and dimethyl carbonate (DMC). | | Aroma threshold values | High strength odor, fishy type; recommend smelling in a 0.01% solution or less. | | General Description | Triethylamine appears as a clear colorless liquid with a strong ammonia to fish-like odor. Flash point 20°F. Vapors irritate the eyes and mucous membranes. Less dense (6.1 lb / gal) than water. Vapors heavier than air. Produces toxic oxides of nitrogen when burned. | | Reactivity Profile | Triethylamine reacts violently with oxidizing agents. Reacts with Al and Zn. Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. | | Health Hazard | Vapors irritate nose, throat, and lungs, causing coughing, choking, and difficult breathing. Contact with eyes causes severe burns. Clothing wet with chemical causes skin burns. Triethylamine may also be irritating to skin and mucous membranes (Windholz et al 1983). | | Fire Hazard | Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. | | Industrial uses | Triethylamine is used as an anti-livering agent for urea- and melamine-based enamels and in the recovery of gelled paint vehicles (HSDB 1988). It is also used as a catalyst for polyurethane foams, a flux for copper soldering, and as a catalytic solvent in chemical synthesis (Hawley 1981). Triethylamine is used in accelerating activators for rubber; as a corrosion inhibitor for polymers; a propellant; wetting, penetrating, and waterproofing agent of quaternary ammonium compounds; in curing and hardening of polymers (i.e. core-binding resins); and as a catalyst for epoxy resins (Hamilton and Hardy, 1974). | | Biochem/physiol Actions | Triethylamine is known to drive polymerization reaction. It acts as a source of carbon and nitrogen for bacterial cultures. Triethylamine is used in pesticides. Triethylamine can serve as an organic solvent. | | Safety Profile | Moderately toxic by
ingestion and skin contact. Mildly toxic by
inhalation. Human systemic effects: visual
field changes. Experimental reproductive
effects. Mutation data reported. A skin and
severe eye irritant. Can cause kidney and
liver damage. A very dangerous fire hazard
when exposed to heat, flame, or oxidizers.
Explosive in the form of vapor when
exposed to heat or flame. Complex with
dinitrogen tetraoxide explodes below 0°C
when undduted with solvent. Exothermic
reaction with maleic anhydride above 150°C.
Can react with oxidzing materials.
Incompatible with N2O4. To fight fire, use
CO2, dry chemical, alcohol foam. When
heated to decomposition it emits toxic
fumes of NOx. | | Potential Exposure | Triethylamine is and aliphatic amine
used as a solvent; corrosion inhibitor; in chemical synthesis;
and accelerator activators; paint remover; base in methylene
chloride or other chlorinated solvents. TEA is used to solubilize
2,4,5-T in water and serves as a selective extractant in
the purification of antibiotics. It is used to manufacture quaternary
ammonia compounds and octadecyloxymethyltriethylammonium
chloride; an agent used in textile treatment. | | Carcinogenicity | TEA was not mutagenic in bacterial assays,
but it did cause aneuploidy and chromosome
aberrations in rats. | | Environmental fate | Photolytic. Low et al. (1991) reported that the photooxidation of aqueous tertiary amine
solutions by UV light in the presence of titanium dioxide resulted in the formation of ammonium
and nitrate ions.
Chemical/Physical. Triethylamine reacted with NOx in the dark to form diethylnitrosamine. In
an outdoor chamber, photooxidation by natural sunlight yielded the following products:
diethylnitramine, diethylformamide, diethylacetamide, ethylacetamide, diethylhydroxylamine,
ozone, acetaldehyde, and peroxyacetyl nitrate (Pitts et al., 1978). | | Metabolism | There have been few studies on the metabolism of industrially important aliphatic amines such as triethylamine. It is generally assumed that amines not normally present in the body are metabolized by monoamine oxidase and diamine oxidase (histaminase).
Ultimately ammonia is formed and will be converted to urea. The hydrogen peroxide formed is acted upon by catalase and the aldehyde formed is thought to be converted to the corresponding carboxylic acid by the action of aldehyde oxidase (Beard and Noe 1981). | | Shipping | UN1296 Triethylamine, Hazard Class: 3; Labels:
3-Flammable liquid, 8-Corrosive material. | | Purification Methods | Dry triethylamine with CaSO4, LiAlH4, Linde type 4A molecular sieves, CaH2, KOH, or K2CO3, then distil it, either alone or from BaO, sodium, P2O5 or CaH2. It has also been distilled from zinc dust, under nitrogen. To remove traces of primary and secondary amines, triethylamine has been refluxed with acetic anhydride, benzoic anhydride, phthalic anhydride, then distilled, refluxed with CaH2 (ammonia-free) or KOH (or dried with activated alumina), and again distilled. Another purification method involved refluxing for 2hours with p-toluenesulfonyl chloride, then distilling. Grovenstein and Williams [J Am Chem Soc 83 412 1961] treated triethylamine (500mL) with benzoyl chloride (30mL), filtered off the precipitate, and refluxed the liquid for 1hour with a further 30mL of benzoyl chloride. After cooling, the liquid was filtered, distilled, and allowed to stand for several hours with KOH pellets. It was then refluxed with, and distilled from, stirred molten potassium. Triethylamine has been converted to its hydrochloride (see brlow), crystallised from EtOH (to m 254o), then liberated with aqueous NaOH, dried with solid KOH and distilled from sodium under N2. [Beilstein 4 H 99, 4 I 348, 4 II 593, 4 III 194, 4 IV 322.] | | Incompatibilities | A strong base. Violent reaction with
strong acids; halogenated compounds; and strong oxidizers.
Attacks some forms of plastics, rubber and coatings.
Corrosive to aluminum, zinc, copper, and their alloys in the
presence of moisture. Reaction with nitrosating agents
(e.g., nitrites, nitrous gases, and nitrous acid) capable of
releasing carcinogenic nitrosamines. | | Waste Disposal | Controlled incineration
(incinerator equipped with a scrubber or thermal unit to
reduce nitrogen oxides emissions). |
| | Triethylamine Preparation Products And Raw materials |
|