| Identification | Back Directory | [Name]
Propoxur | [CAS]
114-26-1 | [Synonyms]
PHC
IPMC
phc7
IMPC
Tugen
Bolfo
oms33
UNDEN
Bifex
PROPER
BAYGON
oms-33
OMS 33
o-Impc
Boruho
Boygon
Brygou
Tendex
Rhoden
Undeen
Undene
DMS 33
PROPOXU
baygone
Sendran
Suncide
Propyon
Isocarb
MITOXUR
bay5122
bay9010
5812315
Aprocarb
UNDEN(R)
BAY 5122
BAY 9010
bay39007
PROPOXUR
PROPOGON
dalfdust
Propotox
Baygon G
Baygon(R)
Pillargon
Propoxure
Dalf Dust
ent25,671
ent-25671
Boruho 50
Blattosep
Mrowkozol
TENDEX(R)
Blattanex
Arporcarb
Arprocarb
BAY 39007
SENDRAN(R)
SUNCIDE(R)
bayerb5122
ENT 25,671
Propoxylor
Propoksuru
Propotox M
phc (jmaf)
Bayer 9010
Invisi-Gard
APROCARB(R)
BAYGON (TM)
BAYER 39007
BLATTANEX(R)
Blattanex 20
Bayer B 5122
chemagro9010
Chemagro 9010
phc(carbamate)
Propoxur (ISO)
Fumite propoxur
Baygon,Propoxur,
Propoxur(Baygon)
BAYGON, 1GM, NEAT
tugonfliegenkugel
Unden (Pesticide)
propoksuru(polish)
Tugon fliegenkugel
propoxur (bsi,iso,esa)
Baygon (TM) 1g [114-26-1]
Isopropoxyphenyl methylcarbamate
o-isopropoxy-phenomethylcarbamate
Methyl-2-isopropoxyphenylcarbamate
o-isopropoxyphenyln-methylcarbamate
2-Isopropoxyphenyl-N-methylcarbamat
2-ISO-PROPOXYPHENYL METHYLCARBAMATE
O-ISOPROPOXYPHENYL METHYL CARBAMATE
o-Isopropoxyphenyl N-methylcarbamate
N-Methyl-2-isopropoxyphenylcarbamate
2-ISOPROPOXYPHENYL-N-METHYL-CARBAMATE
ORTHO-ISOPROPOXYPHENYLMETHYLCARBAMATE
Phenol, o-isopropoxy-, methylcarbamate
PROPOXUR PESTANAL (2-ISOPROPOXY- PHENYL
2-(1-methylethoxy)-phenomethylcarbamate
o-(2-isopropoxyphenyl)n-methylcarbamate
O-(2-Isopropoxyphenyl) N-methylcarbamate
2-(1-Methylethoxy)phenol methylcarbamate
2-(1-Methylethoxy)phenyl methylcarbamate
methyl-carbamicacio-isopropoxyphenylester
n-2-(1-methylethoxy)phenylmethyl-carbamate
2-(1-Methylethoxy)phenyl N-methylcarbamate
phenol,2-(1-methylethoxy)-,methylcarbamate
(2-propan-2-yloxyphenyl) N-methylcarbamate
N-2-(1-Methylethoxy)phenyl methyl-carbamate
n-methylcarbamicacid,o-isopropoxyphenylester
Phenol, 2-(1-methylethoxy)-, methylcarbamate
Methylcarbamic acid, o-isopropoxyphenol ester
BAYGON [N-METHYL-2-ISOPROPOXYPHENYLCARBAMATE]
PROPOXUR (2-ISOPROPOXYPHENYL METHYL CARBAMATE)
2-(1-Methylethoxy)phenol 1-(N-MethylcarbaMate)
Methylcarbamicacid2-(1-methylethoxy)phenylester
methyl-carbamicaci2-(1-methylethoxy)phenylester
Carbamic acid, methyl-, o-isopropoxyphenyl ester
CARBAMICACID,METHYL-,ORTHO-ISOPROPOXYPHENYLESTER
N-methylcarbamic acid (2-isopropoxyphenyl) ester
METHYLCARBAMIC ACID-(1-METHYLETHOXY)PHENYL ESTER
Phenol, 2-(1-Methylethoxy)-, 1-(N-MethylcarbaMate)
n-methylcarbamicacid,2-(1-methylethoxy)phenylester
Carbamic acid, methyl-, 2-(1-methylethoxy)phenyl ester
propoxur (ISO) 2-isopropyloxyphenyl N-methylcarbamate 2-isopropoxyphenyl methylcarbamate | [EINECS(EC#)]
204-043-8 | [Molecular Formula]
C11H15NO3 | [MDL Number]
MFCD00055455 | [MOL File]
114-26-1.mol | [Molecular Weight]
209.24 |
| Chemical Properties | Back Directory | [Appearance]
White, crystalline powder; odorless. Soluble in most alcohols; very slightly solublein water; unstable in highly alkaline media; stableunder normal conditions. | [Melting point ]
91°C | [Boiling point ]
348.6°C (rough estimate) | [density ]
1.1200 | [vapor pressure ]
1.3 x 10-3 Pa (20 °C) | [refractive index ]
1.5080 (estimate) | [Fp ]
-18 °C | [storage temp. ]
2-8°C | [solubility ]
Chloroform: slightly; Methanol: slightly | [form ]
Minute Crystals | [pka]
12.28±0.46(Predicted) | [color ]
White to off-white | [Water Solubility ]
Slightly soluble. 0.2 g/100 mL | [Merck ]
13,7929 | [BRN ]
1879891 | [Exposure limits]
OSHA TWA: 0.5 mg/m3; ACGIH TLV: TWA 0.5 mg/m3. | [CAS DataBase Reference]
114-26-1 | [NIST Chemistry Reference]
2-Isopropoxyphenyl N-methylcarbamate(114-26-1) | [EPA Substance Registry System]
Phenol, 2-(1-methylethoxy)-, methylcarbamate(114-26-1) |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Uses]
Propoxur is a non-systematic carbamate insecticide. Propoxur is used against a wide range of insects such as fleas, mosquitoes, ants, gypsy moths, and other agricultural pests. Propoxur functions by r
eversibly inactivating the enzyme acetylcholinesterase in insects. | [Definition]
ChEBI: A carbamate ester that is phenyl methylcarbamate substituted at position 2 by a propan-2-yloxy group. | [Uses]
Insecticide. | [General Description]
White to tan crystalline powder with a faint, characteristic odor. Used as an insecticide. | [Reactivity Profile]
Propoxur is incompatible with the following: Strong oxidizers, alkalis [Note: Emits highly toxic methyl isocyanate fumes when heated to decomposition.] . | [Hazard]
Toxic by ingestion and inhalation. | [Potential Exposure]
Personnel engaged in the manufacture,
formulation and application of this organonitrogen agricul-
tural chemical and pesticide. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, includ-
ing resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medi-
cal attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit. | [Shipping]
UN2757 Carbamate pesticides, solid, toxic,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
UN2811 Toxic solids, organic, n.o.s., Hazard Class:
6.1; Labels: 6.1-Poisonous materials, Technical Name
Required. | [Incompatibilities]
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explo-
sions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, alkalis, heat, and mois-
ture. Emits highly toxic methyl isocyanate fumes when
heated to decomposition. | [Description]
Propoxur, 2-isopropoxyphenyl methylcarbamate
(IUPAC),forms
colorless crystals, which are moderately soluble in most
organic solvents. | [Waste Disposal]
In accordance with
40CFR165, follow recommendations for the disposal of pes-
ticides and pesticide containers. Must be disposed properly
by following package label directions or by contacting your
local or federal environmental control agency, or by contact-
ing your regional EPA office. Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations govern-
ing storage, transportation, treatment, and waste disposal. | [Health Hazard]
A highly toxic substance by ingestion, andpossibly by most other routes of exposure;moderately toxic by inhalation and skin contact; cholinesterase inhibitor; toxic effects aresimilar to those of other carbamate pesticidesand include excessive salivation, lacrimation, slow heart rate, blurred vision, twitchingof muscle and lack of coordination, nausea,weakness, diarrhea and abdominal pain; oralintake of probably 1.5–3 g could be fatal toadult humans; a teratogenic substance, producing adverse reproductive effects in exper�imental animals.
LD50 oral (rat): 70 mg/kg
LD50 skin (rat): 800 mg/kg
LC50 inhalation (rat): 1440 mg/m3/1 hr. | [Agricultural Uses]
Insecticide, Molluscicide: Not approved for use in EU countries. A non-systemic insecticide compatible with most fungicides and insecticides except those that are alkaline. It is often used in combination with azinphosmethyl, chlorpyrifos, cyfluthrin, dichlorvos, disulfoton or methocarb. It is used on sugar cane, cocoa, pome and stone fruit, grapes, maize, hops, rice, sugar beets, vegetables, cotton, and forestry and ornamentals to control pests such as chewing and sucking insects, ants, crickets, flies, mosquitoes, millepedes, jassids and cockroaches. | [Trade name]
(There are currently 695 registered active and/or canceled and/or transferred products in the U.S.) ARPROCARB®; BAY®; BAY® 5122; BAYER®; BAYER® B 5122; BAYGON®; BIFEX®; BLATTANEX®; BLATTOSEP®; BOLFO®; BO Q 5812-315®; BORUHO®; BORUHO® 50; BRIFUR®; BRYGOU®; CHEMAGRO® 9010; COMPOUND 39007; DALF DUST®; INVISI-GARD®; PILLARGON®; PRENTOX CARBAMATE®; PROPOGON®; PROPOTOX®; PROPOXYLOR®; PROPYON®; RHODEN®; SENDRAN®; SUNCIDE®; TENDEX®; TUGEN®; UNDEN®; UNDENE® | [Environmental Fate]
Groundwater. According to the U.S. EPA (1986) propoxur has a high potential to leach
to groundwater.
Photolytic. Though no products were identified, the half-life in UV irradiated water
(λ >290 nm) was 87.9 hours (Jensen-Korte et al., 1987). When propoxur in ethanol was
irradiated by UV light, only one unidentified cholinesterase inhibitor formed. Exposure
to sunlight for 3 hours yielded no photodecomposition products (Crosby et al., 1965).
Chemical/Physical. Decomposes at elevated temperatures forming methyl isocyanate
(Windholz et al., 1983) and nitrogen oxides (Lewis, 1990). Hydrolyzes in water to 1-
naphthol and 2-isopropoxyphenol (Miles et al., 1988). At pH 6.9, half-lives of 78 and 124
days were reported under aerobic and anaerobic conditions, respectively (Kanazawa,
1987). Miles et al. (1988) studied the rate of hydrolysis of propoxur in phosphate-buffered
water (0.01 M) at 26°C with and without a chlorinating agent (10 mg/L hypochlorite
solution). The hydrolysis half-lives at pH 7 and 8 with and without chlorine were 3.5 and
10.3 days and 0.05 and 1.2 days, respectively (Miles et al., 1988). The reported hydrolysis
half-lives of propoxur in water at pH 8, 9 and 10 were 16.0 days, 1.6 days and 4.2 hours,
respectively (Aly and El-Dib, 1971). In a 0.50 N sodium hydroxide solution at 20°C, the
hydrolysis half-life was reported to be 3.0 days (El-Dib and Aly, 1976). | [Metabolic pathway]
The principal pathways of propoxur metabolism in plants and animals
are deisopropylation, hydrolysis of the carbamate ester to form a phenol
and conjugation. Minor metabolites are formed by hydroxylation of the
N-methyl group and the phenyl ring. Only insects form metabolites
hydroxylated at the isopropoxy group. | [storage]
Store at -20°C | [Degradation]
Propoxur is hydrolysed by strong alkalis. DT50 values at pH 7 and 9
(22°C) were 93 days and 30 hours (PM). Propoxur was hydrolysed
in neutral aerated river water (half-life <2 days at 25 °C) (Kuhr and
Dorough, 1976).
It is not rapidly photodegraded in aqueous solutions (PM) and is more
stable than carbaryl (Crosby et al., 1965). Solutions of unlabelled propoxur
in isopropanol, cyclohexane or cyclohexene were irradiated for 48 hours
with a high-pressure Hg lamp with light of wavelength less than 280 nm filtered out. Analysis of samples was by HPLC. Photodecomposition was
faster in isopropanol than in the hydrocarbon solvents. The main pathway
of photodecomposition (see Scheme 1) was β-cleavage of the carbamate
ester group to form isopropyl phenyl ether (2). 2-Isopropoxyphenol (3)
was detected as a trace product (Schwack and Kopf, 1992). |
| Questions And Answer | Back Directory | [Chemical Properties]
The pure propoxur is white crystalline powder. m.p.91.5℃(industrial products84~87℃) and vapour pressure 1.33Pa (120℃). It can be dissolved in most polar organic solvents, and the solubility in water is 0.2%. It is easy to hydrolyze under alkaline conditions and the half-life of pH at 10 time is 40min (20℃).
| [Uses]
Quick acting, long-acting carbamate insecticide. It has the effect of touch and kill, stomach poison and fumigation, and there is no internal absorption. It is mainly used for prevention and control of rice stem borer and rice leafhopper, planthopper, cotton aphid, scale insect, rust ticks, cereal pests and sanitary insect pest in crops. It can kill in vitro parasites, family health pests (mosquitoes, flies, cockroaches, etc.) and storehouse pests.
| [Toxicity]
The acute oral LD50 is 90 to 128mg/kg for the male rats,104mg/kg for the female rats and 100 to 109mg/kg for the male mice. The acute transdermal LD50 is 800 ~ 1000mg/kg for the rats. In the 2 year feeding test, the non action dose is 800 ~ 1000mg/kg. For carp it is LC50>10mg/L. The acute oral LD50 is 15 ~ 30mg/kg for cowbirds. It is of high toxicity to bees.
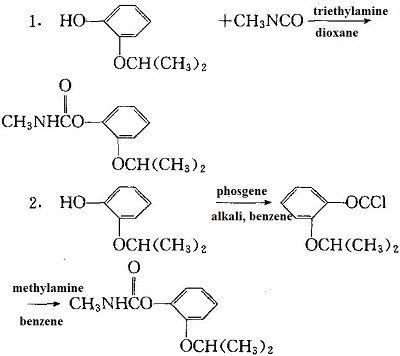
| [Preparation]
The following two methods can be adopted and the reaction is:
|
|
|