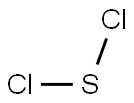| Identification | More | [Name]
Sulfur dichloride | [CAS]
10545-99-0 | [Synonyms]
SULFUR DICHLORIDE
SULFUR (II) DICHLORIDE
SULPHUR DICHLORIDE
SULPHURE BICHLORIDE
U.N. 1828
Chloride of sulfur
Chlorine sulfide
Chlorine sulfide (Cl2S)
chlorinesulfide
Dichloro sulfide
Dichlorosulfane
dichlorosulfoxide
disulfurtetrachloride
Monosulfur dichloride
monosulfurdichloride
SCl2
Sulfur chloride (SCl2)
Sulfur chloride(mono)
Sulfur dichloride (scl2)
Sulfur(II) chloride | [EINECS(EC#)]
234-129-0 | [Molecular Formula]
Cl2S | [MDL Number]
MFCD00011445 | [Molecular Weight]
102.97 | [MOL File]
10545-99-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Reddish-brown, fuming liquid; pungent
chlorine odor. Decomposes in water and
alcohol; soluble in benzene. | [Melting point ]
-122°C | [Boiling point ]
59.6°C (estimate) | [density ]
1.355 g/mL at 25 °C
| [vapor density ]
3.55 (vs air)
| [vapor pressure ]
25.67 psi ( 55 °C)
| [solubility ]
reacts with H2O | [form ]
red viscous liquid | [color ]
red viscous liquid | [Uses]
Chlorine carrier or chlorinating agent, rubber
vulcanizing, vulcanized oils, purifying sugar juices,
sulfur solvent, chloridizing agent in metallurgy,
manufacture of organic chemicals and insecticides. | [CAS DataBase Reference]
10545-99-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Sulfur dichloride(10545-99-0) | [EPA Substance Registry System]
10545-99-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T,N,C | [Risk Statements ]
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed .
R34:Causes burns.
R40:Limited evidence of a carcinogenic effect.
R50:Very Toxic to aquatic organisms.
R37:Irritating to the respiratory system.
R14:Reacts violently with water.
R35:Causes severe burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S27:Take off immediately all contaminated clothing .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [RIDADR ]
UN 3390 6.1/PG 1
| [HazardClass ]
8 | [PackingGroup ]
I | [HS Code ]
28121042 | [Safety Profile]
Poison irritant and corrosive to shin, eyes, and mucous membranes. Flammable when exposed to heat or flame. Reacts violently with Al, NH3, K, Na, acetone, dunethyl sulfoxide, water, oxidants, metals, hexafluoroisopropylidene amino lithium, and toluene. Reactive with water or steam. When heated to decomposition it emits very toxic fumes of SOx and Cl-. | [Hazardous Substances Data]
10545-99-0(Hazardous Substances Data) |
| Hazard Information | Back Directory | [Chemical Properties]
Reddish-brown, fuming liquid; pungent
chlorine odor. Decomposes in water and
alcohol; soluble in benzene. | [Hazard]
Toxic by inhalation and ingestion, strong
irritant to tissue. | [Purification Methods]
Distil sulfur chloride twice in the presence of a small amount of PCl3 through a 12in Vigreux column (p 11), the fraction boiling between 55-61o being redistilled (in the presence of PCl3), and the fraction distilling between 58-61o retained. (The PCl3 is added to inhibit the decomposition of SCl2 into S2Cl2 and Cl2). The SCl2 must be used as quickly as possible after distillation — within 1hour at room temperature. The sample contains 4% of S2Cl2. On long standing this reaches 16-18%. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 371-372 1963.] HARMFUL VAPOURS. |
|
|