| Identification | More | [Name]
Tamoxifen | [CAS]
10540-29-1 | [Synonyms]
TAMOXIFEN
TAMOXIFEN BASE
TRANS-2-[4-(1,2-DIPHENYL-1-BUTENYL)PHENOXY]-N,N-DIMETHYLETHYLAMINE
[Z]-1-[P-DIMETHYLAMINOETHOXYPHENYL]-1,2-DIPHENYL-1-BUTENE
(Z)-2-[4-(1,2-DIPHENYL-1-BUTENYL)PHENOXY]-N,N-DIMETHYLETHANAMINE
(z)-2-(4-(1,2-diphenyl-1-butenyl)phenoxy)phenoxy)-n,n-dimethylethanamine
(z)-2-(para-(1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethylamine
1-para-beta-dimethylaminoethoxyphenyl-trans-1,2-diphenylbut-1-ene
1-p-beta-dimethylaminoethoxyphenyl-trans-1,2-diphenylbut-1-ene
ici47699
n-dimethyl-2-(p-(1,2-diphenyl-1-butenyl)phenoxy)-(z)-ethylamin
Nolvadex-D
tamoxifen(z)
tamoxifendrugstandardsolution
trans-tamoxifen
z-tamoxifen
Genox
Tamoxen
tamoxifen free base
Ethanamine, 2-4-(1Z)-1,2-diphenyl-1-butenylphenoxy-N,N-dimethyl- | [EINECS(EC#)]
234-118-0 | [Molecular Formula]
C26H29NO | [MDL Number]
MFCD00010454 | [Molecular Weight]
371.51 | [MOL File]
10540-29-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
97-98 °C(lit.)
| [Boiling point ]
501.18°C (rough estimate) | [density ]
1.0630 (rough estimate) | [vapor pressure ]
0Pa at 25℃ | [refractive index ]
1.6000 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
H2O: insoluble <0.1% at 20°C | [form ]
neat | [pka]
pKa 8.71(H2O
t = 25
I = 0.025) (Uncertain) | [color ]
Crystals from pet ether | [Water Solubility ]
Insoluble in water. Soluble in methanol, ethanol, propanol or propylene glycol.Soluble in dimethyl sulfoxide, dichloromethane and ethanol. Insoluble in water. | [Usage]
A nonsteroidal estrogen antagonist of interest in the treatment of some forms of breast cancer. Tamoxifen is a Protein Kinase C inhibitor, and induces apoptosis in human malignant glioma cell lines | [Merck ]
13,9137 | [Stability:]
Light Sensitive | [InChIKey]
NKANXQFJJICGDU-QPLCGJKRSA-N | [LogP]
6.3 at 20℃ | [CAS DataBase Reference]
10540-29-1(CAS DataBase Reference) | [IARC]
1 (Vol. 66, 100A) 2012 | [EPA Substance Registry System]
10540-29-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xi | [Risk Statements ]
R45:May cause cancer.
R60:May impair fertility.
R61:May cause harm to the unborn child.
R64:May cause harm to breast-fed babies.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [RTECS ]
KR5919600
| [HS Code ]
29221990 | [Safety Profile]
Confirmed human
carcinogen. Moderately toxic by ingestion
and intraperitoneal routes. Human systemic
effects by an unspecified route: nausea or
vomiting, leukopenia, thrombocytopenia,
and skin changes. An experimental
teratogen. Other experimental reproductive
effects. Human mutation data reported.
When heated to decomposition it emits
toxic fumes of NOx. | [Hazardous Substances Data]
10540-29-1(Hazardous Substances Data) | [Toxicity]
LD50 orl-rat: 4100 mg/kg DRFUD4 9,186,84 |
| Hazard Information | Back Directory | [Description]
In 1966, ICI Pharmaceuticals (now AstraZeneca) first synthesized
tamoxifen in the hope of developing a morning-after
contraceptive pill. The UK patent for this compound was in
place in 1962, whereas the US patent was repeatedly denied
until the 1980s. Tamoxifen was approved for a fertility treatment
but it was not proven as useful in regulating human
contraception. Even though there was a link between estrogen
and breast cancer, developing a cancer treatment was not
a priority at the time. In 1971, the first clinical study showed
a convincing effect of tamoxifen in treating advanced breast
cancer. From 1971 to 1977, this drug was neither clinically nor
financially remarkable. In 1980s, however, publications first
showed that tamoxifen, in addition to chemotherapy,
improved survival for patients with early stage breast cancer. In
1998, the meta-analysis by the Oxford-based Early Breast
Cancer Trialists’ Collaborative Group showed that tamoxifen
did indeed save lives in early breast cancer. In 2001, tamoxifen
sales were over $1.024 billion. Since the expiration of the
patent in 2002, it is now widely available as a generic drug. By
2004, tamoxifen was the best selling hormonal drug for the
treatment of breast cancer. | [Chemical Properties]
White Crystalline Solid | [Originator]
Nolvadex,I.C.I.,UK,1973 | [Definition]
ChEBI: Tamoxifen is a tertiary amino compound and a stilbenoid. It has a role as an estrogen receptor antagonist, a bone density conservation agent, an estrogen receptor modulator, an estrogen antagonist, an angiogenesis inhibitor, an EC 2.7.11.13 (protein kinase C) inhibitor, an EC 1.2.3.1 (aldehyde oxidase) inhibitor and an antineoplastic agent. It derives from a hydride of a stilbene. | [Indications]
Tamoxifen (Nolvadex) is a synthetic antiestrogen used in the treatment of breast cancer.
Normally, estrogens act by binding to a cytoplasmic protein
receptor, and the resulting hormone–receptor complex
is then translocated into the nucleus, where it induces
the synthesis of ribosomal RNA (rRNA) and messenger
RNA (mRNA) at specific sites on the DNA of the target
cell. Tamoxifen also avidly binds to estrogen receptors and
competes with endogenous estrogens for these critical sites.
The drug–receptor complex has little or no estrogen agonist
activity.Tamoxifen directly inhibits growth of human
breast cancer cells that contain estrogen receptors but has
little effect on cells without such receptors. | [Indications]
Tamoxifen is a partial estrogen agonist in breast and
thus is used as a treatment and chemopreventative for
breast cancer. Tamoxifen is a full agonist in bone and
endometrium, and prolonged use of tamoxifen leads to
a fourfold to fivefold increase in the incidence of endometrial
cancer. See Chapter 56 for a detailed discussion
of the use of tamoxifen in breast cancer. | [Manufacturing Process]
To the Grignard reagent prepared from 0.59 part of magnesium, 3.95 parts of
bromobenzene and 50 parts of ether there are added 7.5 parts of 4-(β-
dimethylaminoethoxy)-α-ethyldesoxybenzoin in 50 parts of ether. After
heating under reflux for 3 hours, the mixture is decomposed by the addition
of a solution of 60 parts of ammonium chloride in 150 parts of water. The
mixture is separated, and the ethereal layer is dried with anhydrous sodium
sulfate, and the ether is evaporated. The residue is crystallized from
methanol. There is thus obtained 1-(p-β-dimethylaminoethoxyphenyl)-1,2-
diphenylbutan-1-ol, melting point 120°C to 121°C.
2.15 parts of 1-(p-β-dimethylaminoethoxyphenyl)-1,2-diphenylbutan-1-ol, 25
parts of ethanol and 0.8 part of 10 N hydrochloric acid are heated together
under reflux for 3 hours. The solution is evaporated to dryness under reduced
pressure and the residue is extracted with methylene chloride. The methylene
chloride extract is decolorized with charcoal and then evaporated to dryness.
The residue is dissolved in 100 parts of water, the solution is basified by the
addition of sodium hydroxide solution, and the precipitated solid is extracted
three times, each time with 50 parts of ether. The combined extracts are dried with anhydrous sodium sulfate and then evaporated. The residue is
crystallized from aqueous methanol, and there is thus obtained 1-(p-β-
dimethylaminoethoxyphenyl)-1,2-diphenylbut-1-ene, melting point 95°C to
96°C. | [Brand name]
Nolvadex (AstraZeneca); Soltamox (Savient). | [Therapeutic Function]
Antiestrogen, Antineoplastic | [World Health Organization (WHO)]
Tamoxifen is an anti-estrogen agent used mainly to treat breast
cancer. Tamoxifen is listed in the WHO Model List of Essential Drugs. | [General Description]
Tamoxifen is a selective estrogen response modifier (SERM), protein kinase C inhibitor and anti-angiogenetic factor. Tamoxifen is a prodrug that is metabolized to active metabolites 4-hydroxytamoxifen (4-OHT) and endoxifen by cytochrome P450 isoforms CYP2D6 and CYP3A4. In breast cancer, the gene repressor activity of tamoxifen against ERBB2 is dependent upon PAX2. Blocks estradiol-stimulated VEGF production in breast tumor cells. | [Biochem/physiol Actions]
Protein kinase C inhibitor. Induces apoptosis in human malignant glioma cell lines. Tamoxifen and its metabolite 4-hydroxytamoxifen are selective estrogen response modifiers (SERMs) that act as estrogen antagonists in mammary gland. Blocks estradiol-stimulated VEGF production in breast tumor cells. | [Mechanism of action]
Tamoxifen is slowly absorbed, and maximum serum
levels are achieved 4 to 7 hours after oral administration.
The drug is concentrated in estrogen target tissues, such
as the ovaries, uterus, vaginal epithelium, and breasts.
Hydroxylation and glucuronidation of the aromatic
rings are the major pathways of metabolism; excretion
occurs primarily in the feces. | [Clinical Use]
Tamoxifen is a SERM that is used as an antiestrogen in the treatment of estrogen-dependent breas Tcancer following prim ary treatment (c hemotherapy and/or surgery). | [Synthesis]
Tamoxifen, (Z)-2-[p-(1,2-diphenyl-1-butenyl)phenoxy]N,N-dimethylethylamine
(28.2.8), is synthesized from |á-ethyldezoxybenzoin. Interaction of this with 4-
methoxyphenylmagnesium bromide gives the corresponding carbinol (28.2.5). Its
dehydration in acidic conditions gives a derivative of stilbene (28.2.6), and further heating
of which with quinidine hydrochloride as a demethylating agent gives 2-[p-(1,2-diphenyl-
1-butenyl)phenol] (28.2.7). The phenolic hydroxyl is further alkylated by dimethylaminoethylchoride
using sodium ethoxide as a base, which forms a mixture of E and Z
isomers of the final product. The desired Z isomer, tamoxifen (28.2.8) is isolated by fractional
crystallization from petroleum ester.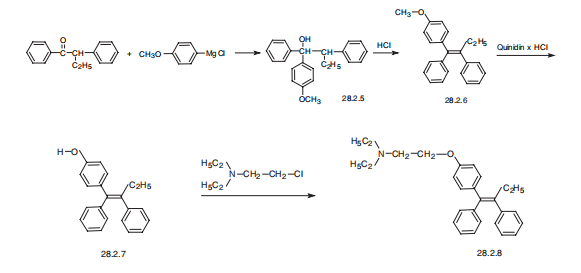
| [in vitro]
ic50s for growth inhibition ranged from 5.5–10 μm, and were not affected by estrogen. tamoxifen-mediated growth inhibition was not associated with induction of tgf-β. however, tamoxifen treatment was associated with inhibition of pkc, which was followed by induction of p21waf1/cip1, rb dephosphorylation, and g1/s phase cell cycle arrest [1]. | [in vivo]
the tumor cell kinetics of mcf-7 human breast carcinoma xenografts grown in nude mice can be significantly altered by hormonal manipu lation. tamoxifen treatment or e2 deprivation resulted in an approximate doubling of the tpol and an approximately 40% reduction in labeling index as compared to e2-stimulated tumors. an increase in cell loss rate was calculated for both tamoxifen treatment and e2 deprivation [2]. | [IC 50]
5.5–10 μm | [Carcinogenicity]
Tamoxifen is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans. | [Metabolism]
Tamoxifen is extensively metabolised by cytochrome
P450 isoenzymes, to active metabolites that include
N-desmethyltamoxifen, 4-hydroxytamoxifen, and
4-hydroxy-N-desmethyltamoxifen (endoxifen).
Metabolism is by hydroxylation, demethylation and
conjugation.
In-vitro studies suggest that both N-desmethyltamoxifen
and 4-hydroxytamoxifen are further metabolised to
endoxifen.
Elimination occurs, chiefly as conjugates with practically
no unchanged drug, principally through the faeces and to
a lesser extent through the kidneys. | [storage]
Room temperature | [References]
[1] rohlff c, blagosklonny mv, kyle e, kesari a, kim iy, zelner dj, hakim f, trepel j, bergan rc. prostate cancer cell growth inhibition by tamoxifen is associated with inhibition of protein kinase c and induction of p21(waf1/cip1). prostate. 1998 sep 15;37(1):51-9.
[2] jann n. sarkaria, david f. c. gibson, v. craig jordan, john f. fowler, mary j. lindstrom, andr. timothy mulcahy. tamoxifen-induced increase in the potential doubling time of mcf-7 xenografts as determined by bromodeoxyuridine labeling and flow cytometry. cancer research 5.1. 4413-1417, september 15, 1993.
[3] osborne ck. tamoxifen in the treatment of breast cancer. n engl j med. 1998 nov 26;339(22):1609-18. |
| Questions And Answer | Back Directory | [Antiestrogen drug]
Tamoxifen is non-steroidal anti-estrogen drugs. Its structure is similar to estrogen, existing Z type and O type isomers. The physical and chemical properties are different from each other, and physiological activity is different. E type has weak estrogenic activity, Z type having the effect of anti-estrogen. If the estrogen receptor (ER) is present in breast cancer cells, estrogen enters into tumor cells and binds with ER, promoting mRNA and DNA synthesis of tumor cells, stimulating tumor cell growth. However, Tamoxifen Z isomer enters into the cell, competitively binding with ER to form receptor complexes, inhibiting that estrogen plays an role, and inhibiting proliferation of breast cancer cells. Clinically it is mainly used for high levels of estrogen in breast cancer patients, which combines with androgen and other anticancer drugs (such as doxorubicin, etc., enhancing the effectiveness and showing good effect in postmenopausal patients with advanced breast cancer. Oral: once 10~20mg, 2 times 1 day. Common side effects are flushing, genital itching, occasional vaginal bleeding, a few may have a headache, fluid retention, for a long time may have retinal disease, vision loss, the other can have bone marrow suppression and gastrointestinal reactions.
Tamoxifen is used to treat breast cancer, and can reduce mortality and recurrence rate of estrogen-dependent breast cancer patients, so it has a good prospect. Another endocrine therapy aromatase inhibitor, it can inhibit effect of aromatase, preventing that the and rostenedione secreted by adrenal gland is transformed into estrogen in peripheral tissues (fat, skin, muscle), further reducing estrogen levels in postmenopausal women. It is currently one of the important means to treat postmenopausal patients with estrogen and progesterone receptor-positive breast cancer.
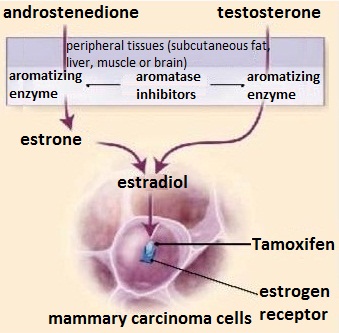
Figure 1 Mechanism of action of Tamoxifen and aromatase inhibitors | [Origin of the study]
In the 1960s, as for initial research of estrogen drug, scientists found that anti-estrogen drugs can prevent endometrial hyperplasia and embryo implantation in rats, and has the role of contraception, hoping that it can be used as contraceptives for use in humans. In1966 UK reproductive endocrinologist Walpole firstly reported study of Tamoxifen. When investigating an anti-estrogen substance-tristyrylphenol, they found that its two derivatives had different physiological effects. They used vaginal epithelium keratosis index and uterine weight growth index as the evaluation index. Results showed that homeopathic structure (ICI47,699) has estrogen-like effects on uterus and vaginal epithelial cells in rat and mice; trans structure (ICI46,747) has very weak estrogenic effect on vaginal epithelial cells in rat, has an anti-estrogenic effect on the uterus, which can terminate early pregnancy and inhibit ovulation, but it also has anti-estrogenic effect on uterus and vaginal epithelial cells in mouse. Trans structure ICI46,747 which is now Tamoxifen, scientists expect that ICI46,747 can be used as a new contraceptives in humans. However, during the clinical trial, they found that the drug did not show the same effect in rats, but the drug can stimulate endometrial hyperplasia, and promote ovulation. Thus, clinically Tamoxifen was firstly used as ovulation drugs in anovulatory infertility patients, and still in use. For the current field of assisted reproductive technology treatment of ovarian hyperstimulation program, it also has a good effect on ovulation. Thus, Tamoxifen had both estrogen-like and anti-estrogenic effects, and showed different effects on different species and tissues.
Figure 2 The structural formula of Tamoxifen.
The above information is edited by the Chemicalbook of Liu Yujie.
| [Uses]
1. Treatment for Women with metastatic breast cancer recurrence.
2. Used as adjuvant therapy after surgery for breast cancer metastasis, and relapse prevention.
3. For the treatment of ovarian cancer, endometrial cancer and endometriosis.
| [Pharmacokinetics]
This product is easily absorbed orally, generally three hours after taking the drug concentration in the blood is up to the peak; because of enterohepatic circulation, the concentration of product is a more lasting in vivo; in the liver metabolism this product mainly excreted in the feces by biliary (58% to 100% ) rarely excreted in urine (only 2% to 21%); after the anima taking isotopically labeled this product, it is found that radioactivity is the highest in animal ovaries.After it is used for post-menopausal women, the concentration in endometrium is 2 times in plasma . | [Side effects]
1. Early treatment of bone cancer pain and may be a transient increase, continued treatment can be gradually reduced.
2. gastrointestinal reactions: loss of appetite, nausea, vomiting, diarrhea.
3. Reproductive system: menstrual disorders, amenorrhea, vaginal bleeding, genital itching, endometrial hyperplasia, endometrial polyps and endometrial cancer.
4. Skin: facial flushing, rash, hair loss.
5. Bone marrow: occasionally neutropenia and thrombocytopenia.
6. Liver function: occasionally abnormalities.
7. Eyes: using for long time (17 months or more) and large number (240~320mg per day), may appear retinal lesions or corneal opacity .
8. The rare and needed attention of side effects: insanity, pulmonary embolism (showing shortness of breath), thrombosis, weakness, drowsiness. | [Contraindications]
1. It is contraindicated in patients who are allergic to this product.
2. It is contraindicated in patients with fundus diseases.
3. It is contraindicated in patients who have history of deep vein thrombosis and pulmonary embolism or are receiving anticoagulant therapy.
4. It is contraindicated in pregnant woman and nursing mothers. | [Precautions]
1. Abnormal liver function should be used with caution. If bone metastasis, patients are needed to regularly check blood calcium in the early treatment.
2.Pregnancy safety of this drug is classified as D class by FDA. | [Drug interactions]
1. The drug combines with fluorouracil, cyclophosphamide, methotrexate, vincristine and doxorubicin, etc. and can improve the effects.
2. The drug can increase the dopaminergic effect of bromocriptine mesylate.
3. The data show that the drug can prolong neuromuscular blockade of atracurium.
4. The drug can enhance the effect of anticoagulants, not combining with anticoagulants (such as warfarin, two coumarin anticoagulants).
5. Antacids and cimetidine, famotidine, ranitidine can change the pH of the stomach, making the drug enteric-coated tablets decomposed and showing a stimulating effect on the stomach, so when l in combination , these drugs should be interval of 1 to 2 hours.
6. Estrogen can affect the therapeutic effect of the drug, should not be combined.
7. The in vitro test results show that the drug may inhibit the metabolism of tacrolimus.
8.When in combination with mitomycin, the risk of hemolytic syndrome and hematuria increased.
9. The drug combines with triptolide which can lead to accelerate tumor growth in mice, so the combination should be cautious.
10. with allopurinol, the drug may increase liver toxicity.
11. The drug combines with other cytotoxic drugs, increasing the risk of thromboembolism. |
|
|