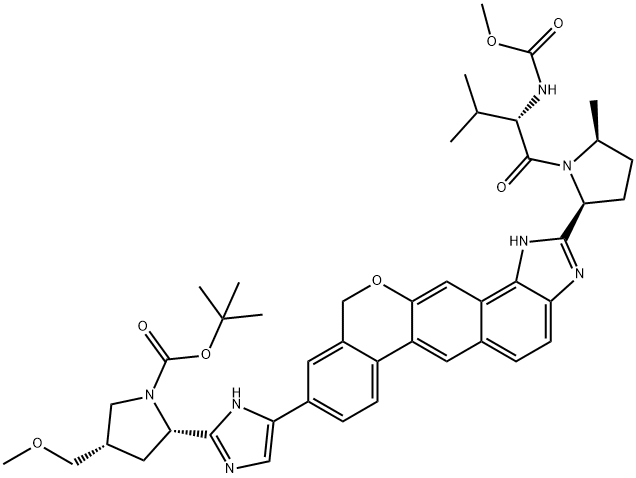
Velpatasvir intermediate synthesis
- Product Name:Velpatasvir intermediate
- CAS Number:1378391-45-7
- Molecular formula:C44H53N7O7
- Molecular Weight:791.93
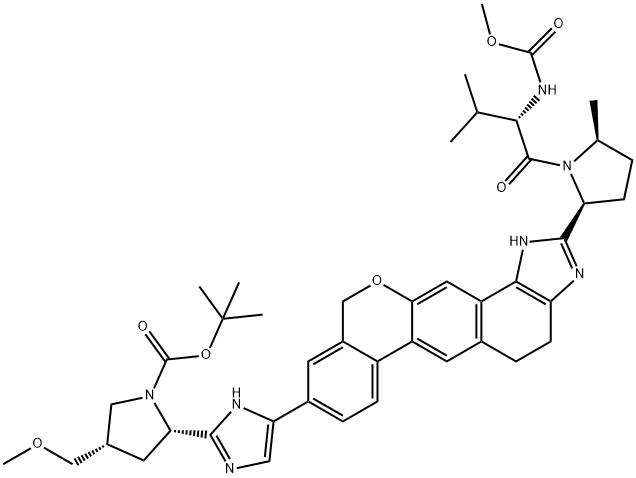
tot-Butyl (2S,4S)-2-[5-(2-{(2S,5S)-l-[N-(methoxycarbonyl)-L-valyl]-5-methylpyrrolidin-2-yl}- l,ll-dihydroisochromeno[4',3':6,7]naphtho[l,2-d]imidazol-9-yl)-lH-imidazol-2-yl]-4- (methoxymethyl)pyrrolidine-l-carboxylate tert-Butyl (2S,4S)-2-[5-(2- {(2S,5S)-l-[N-(methoxycarbonyl)-L-valyl]-5-methylpyrrolidin-2- yl} -l ,4,5,l l-tetrahydroisochromeno[4',3':6,7]naphtho[l,2-d]imidazol-9-yl)-lH-imidazol-2-yl]-4- (methoxymethyl)pyrrolidine-l-carboxylate (8.33 g, 1.049 mmol) was suspended in DCM and activated Mn02 (55.0 g, 630 mmol) was added in a single portion. After 13 h, MeOH (200 mL) was added and the slurry was filtered over celite. The filter cake was washed with MeOH (600 mL) and the filtrate was concentrated under reduced pressure. The crude material was purified by silica column chromatography (0% to 45% MeOH/EtOAc) to afford tert-butyl (2S,4S)-2-[5-(2- {(2S,5S)-l-[N- (methoxycarbonyl)-L-valyl] -5 -methylpyrrolidin-2-yl} -1,11- dihydroisochromeno[4',3':6,7]naphtho[l,2-d]imidazol-9-yl)-lH-imidazol-2-yl]-4- (methoxymethyl)pyrrolidine-l-carboxylate (4.85 g, 58%).
1378391-86-6
4 suppliers
inquiry
![Methyl [(2S)-3-methyl-1-[(2S,5S)-2-methyl-5-[9-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,11-dihydroisochromeno[4',3':6,7]naphtho[1,2-d]imidazol-2-yl]pyrrolidin-1-yl]-1-oxobutan-2-yl]carbamate](/CAS/20210111/GIF/1378392-97-2.gif)
1378392-97-2
6 suppliers
inquiry

1378391-45-7
110 suppliers
inquiry
Yield:1378391-45-7 730 mg
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;tetrakis(triphenylphosphine) palladium(0);potassium carbonate in 1,4-dioxane;dimethyl sulfoxide at 95; for 5 h;
Steps:
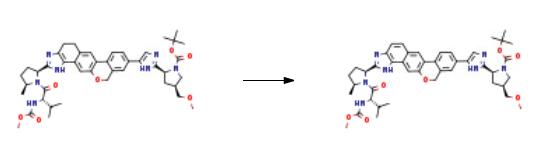
5.2
Step 2: In a mixture of 20 ml DMSO and 20 ml dioxane, add 1.25 g i-1 and 0.88 g III, and stir to dissolve;Then 230 mg of Pd(PPh3)4, 144 mg of PdCl2(dPPf)2 and 3.2 ml of 2M K2CO3 solution were successively added.The reaction solution was degassed with N2 for 10 min and heated to 95° C. for 5 h. Cool the reaction solution to room temperature and add ethyl acetate. Saturated NaHCO3, brine washing; the organic phase was dried over anhydrous MgSO4 overnight, filtered, concentrated under reduced pressure, and chromatographed on a silica gel column. Separated and eluted with a mixed solvent of methanol:ethyl acetate (1:9). The desired fractions were collected and evaporated to dryness under reduced pressure to give N-(tert-butyloxycarbonyl). Based on (2S,4S)-2-[5-(2-{(2S,4S)-1-[N-(methoxycarbonyl)-L-prolyl]-5-methylpyrrolidine- 2-yl}-1,11-dihydroisochromen[4',3':6,7]naphtho[1,2-d]imidazol-9-yl)-1H-imidazol-2-yl]- 4-(Methoxymethyl)pyrrolidine (i-2) 730 mg.
References:
CN107556324,2018,A Location in patent:Paragraph 0041; 0042; 0044
1378391-44-6
32 suppliers
inquiry

1378391-45-7
110 suppliers
inquiry
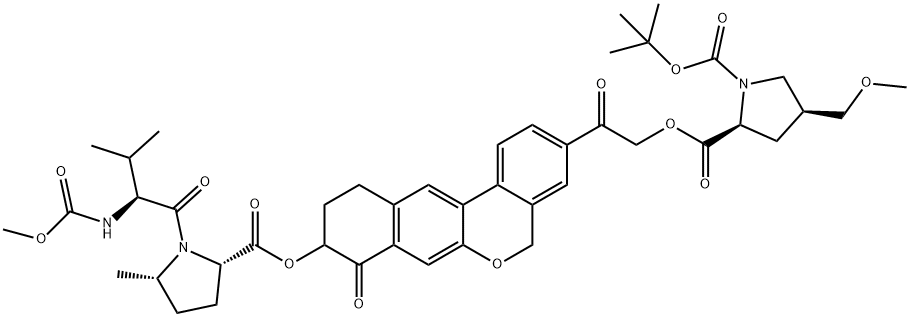
1378391-43-5
35 suppliers
inquiry

1378391-45-7
110 suppliers
inquiry
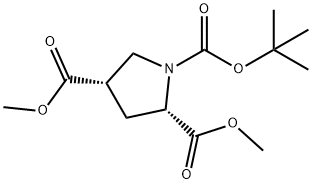
1378388-30-7
6 suppliers
inquiry

1378391-45-7
110 suppliers
inquiry
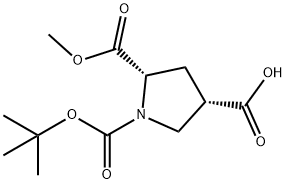
1378388-35-2
43 suppliers
inquiry

1378391-45-7
110 suppliers
inquiry