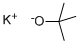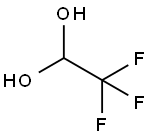
TRIFLUOROACETALDEHYDE HYDRATE synthesis
- Product Name:TRIFLUOROACETALDEHYDE HYDRATE
- CAS Number:421-53-4
- Molecular formula:C2H3F3O2
- Molecular Weight:116.04
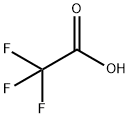
76-05-1
713 suppliers
$9.00/1g

421-53-4
149 suppliers
$15.00/1g
Yield:-
Reaction Conditions:
with lithium aluminium tetrahydride at 20; for 15 h;
Steps:
15.a
a) A solution of 2,2,2-trifluoroacetic acid (68) (6.0 g, 52.6 mmol) in diethyl ether (80 ml) was added dropwise over 80 minutes to a suspension of lithium aluminiumhydride (1.52 g, 40.0 mmol) in diethyl ether (100 ml) at 0° C. and stirred at room temperature for 15 hours. The reaction was quenched with methanol (3.6 ml), H2O (3.2 ml) and concentrated H2SO4 (6.4 ml) and the resulting precipitation was filtered off. The filtrate was washed with H2O and brine, dried over MgSO4, filtered and concentrated in vacuo to give a crude product of 2,2,2-trifluoroethane-1,1-diol (69). This was diluted with methanol (10 ml) and ice-water (20 g), and then hydroxylamine hydrochloride (4.06 g, 58.4 mmol) and aqueous NaOH solution (50%, 8.8 g, 110 mmol) was added successively to the mixture. The reaction mixture was stirred at room temperature for 16 hours and washed with diethyl ether (50 ml). Thus obtained aqueous phase was neutralized (pH=6) with concentrated hydrochloric acid, extracted with diethyl ether (100 ml×3), washed with H2O and brine, dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by distillation to give (E)-2,2,2-trifluoroacetaldehyde oxime (70) (4.94 g, yield 53%) as a colorless oil (bp: 75° C.).
References:
Shinogi & Co., Ltd. US2011/136833, 2011, A1 Location in patent:Page/Page column 37
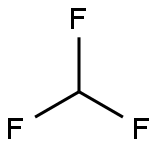
75-46-7
0 suppliers
$150.00/50 g

421-53-4
149 suppliers
$15.00/1g
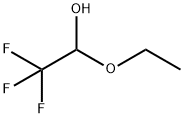
433-27-2
146 suppliers
$12.00/1g

421-53-4
149 suppliers
$15.00/1g