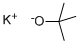
Potassium tert-butoxide synthesis
- Product Name:Potassium tert-butoxide
- CAS Number:865-47-4
- Molecular formula:C4H9KO
- Molecular Weight:112.21
75-65-0
752 suppliers
$10.00/10ml
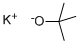
865-47-4
678 suppliers
$14.00/25g
Yield:-
Reaction Conditions:
with potassium amalgam;catalyst comprising porous iron for 2.2 h;Conversion of starting material;
Steps:
2
A bed of 500 ml of the catalyst from example 1 was placed in a glass reactor (upright tube). Potassium amalgam (0.411% by weight of K in Hg) was passed through this bed from the top downward at a flow rate of 6 l/h. At the same time, 400 g of t-butanol were circulated through the column at a flow rate of 10 l/h by pumping. The progress of the reaction is determined by acid-based titration of samples of the alcohol phase. The reaction was complete after 2.2 hours. The potassium tert-butoxide solution was drained and the amount of potassium tert-butoxide produced was found to be 0.76 mol by acid-based titration. The amount of potassium hydroxide formed as by-product was determined by means of Karl-Fischer titration. The calculated space-time yield of potassium tert-butoxide is 77 g/h*l.
References:
US2005/101806,2005,A1 Location in patent:Page/Page column 4