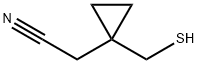
Thiomethyl Cyclopropyl Acetonitrile synthesis
- Product Name:Thiomethyl Cyclopropyl Acetonitrile
- CAS Number:866923-64-0
- Molecular formula:C6H9NS
- Molecular Weight:127.21
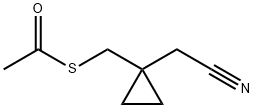
152922-72-0
13 suppliers
inquiry

866923-64-0
11 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 1-(Acetylthiomethyl)cyclopropaneacetonitrilewith methanol;sodium methylate at 10; for 3 h;
Stage #2: with water;acetic acid in toluene at 0 - 5; pH=~ 4;
Steps:
19
EXAMPLE 19: PREPARATION OF (I-MERCAPTOMETHYL-CYCLOPROPYL)- ACETONITRILE (FORMULA XIV):1-(acetylthiomethyl)-cyclopropaneneacetonitrile (33 kg) and methanol (82 liters) were taken into a reactor and cooled to 10 0C. Sodium methoxide solution of concentration of about 20% (58.3 kg) was added slowly into the reaction mass at about 10 0C followed by addition of methanol (42 liters). The reaction mass was maintained at 10 0C for about 3 hours and decomposed slowly by addition of water (330 liters). N- heptane (165 liters) was then added followed by stirring for about 15 minutes at 30 0C. Separated the layers and washed the aqueous layer with 4x165 L of n-heptane. The aqueous layer was taken into a fresh reactor containing toluene (198 liters). Cooled the reaction solution to 0 0C, and then pH of the reaction mass was adjusted to about 4 with acetic acid (42 liters) at below 5 0C. The organic layer was separated and the aqueous layer was extracted into toluene (2x132 liters). The combined organic layer was washed with sodium bicarbonate solution (6.6 kg of sodium bicarbonate dissolved in 132 L of water) in two equal lots followed by washing with water (3χ132 liters). The separated organic layer was treated with activated carbon (4.95 kg) and maintained for about 30 minutes. Filtered the reaction mass through a leaf filter and washed the cake with toluene (66 liters). The reaction solution was distilled off completely under vacuum to 80% of the original volume. The obtained residue was cooled to about 30 °C and charged into an agitated thin film evaporator followed by heating to 550C under a vacuum of 700 mmHg. The obtained crude was cooled to 30 0C to obtain 18.4 liters of EPO
References:
WO2008/58118,2008,A2 Location in patent:Page/Page column 37-38

152922-72-0
13 suppliers
inquiry
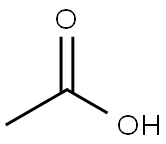
64-19-7
1601 suppliers
$10.00/25ML

866923-64-0
11 suppliers
inquiry