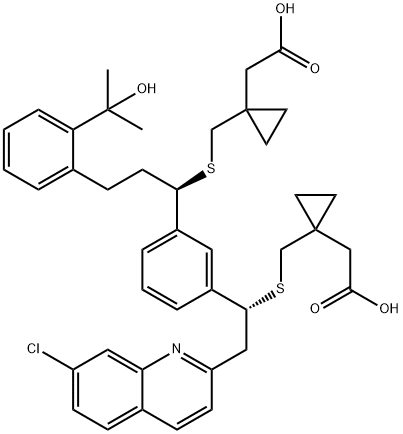
Montelukast Bis-sulfide (Mixture of Diastereomers) synthesis
- Product Name:Montelukast Bis-sulfide (Mixture of Diastereomers)
- CAS Number:1187586-61-3
- Molecular formula:C41H46ClNO5S2
- Molecular Weight:732.39
![2-[1-(Mercaptomethyl)cyclopropyl]acetic acid](/CAS/GIF/162515-68-6.gif)
162515-68-6
380 suppliers
$11.00/1g
151767-02-1
708 suppliers
$8.00/50mg

1187586-61-3
71 suppliers
inquiry
1187586-58-8
63 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 1-(sulfanylmethyl)cyclopropaneacetic acidwith sodium t-butanolate;PEG-600 in toluene;Inert atmosphere;
Stage #2: Montelukast sodium in tetrahydrofuran;toluene at 20;Inert atmosphere;
Steps:
12
EXAMPLE 12 (Preparation of the standards of 2-[(R)-l-[[[l-[3-[2-(7-chloro-2-quinolinyl)- (S)- 1 -( { [ 1 -(carboxymethyl)cyclopropyl]methyl} thio)ethyl]phenyl]-3-[2-( 1 -hydroxy- 1 - methylethyl)phenyl]-propyl]thio]methyl]cyclopropane]acetic acid and 2-[(R)-l-[[[l-[3-[2-(7- chloro-2-quinolinyl)-(R)-l-({[l-(carboxymethyl)cyclopropyl]-methyl}thio)ethyl]phenyl]-3-[2- (l-hydroxy-l-methyl-ethyl)phenyl]propyl]thio]methyl]cyclopropane]acetic acid)To [l-(mercaptomethyl)cyclopropyl] acetic acid (0.7 g) toluene (20 ml), sodium tert-butoxide (0.85 g) and a solution of 2.6 g of PEG-600 in 3 ml of toluene were added under argon atmosphere. Then, a solution of montelukast sodium (2.72 g) in 15 ml of tetrahydrofuran was added dropwise to the stirred mixture. The obtained mixture was stirred at the laboratory temperature and under argon atmosphere for 30 days. Then, toluene (100 ml) was added and 45 ml of the liquid was removed by vacuum distillation. The residue was washed with a solution of tartaric acid and water. The organic phase was dried over sodium sulfate and concentrated to the volume of 30 ml after filtration of the desiccant. To the concentrated residue 3 ml of acetonitrile, 0.5 ml of isopropylamine and gradually 30 ml of heptane were added. The separated suspension of the salt of montelukast with isopropylamine was filtered off and the filtered mother liquor was concentrated in vacuum. 0.4 g of an oily product were obtained, which, according to HPLC, contained 70% of a mixture of montelukast diastereoisomers I and II. The standards of both the diastereoisomers in the form of free acids were obtained in the quantities of approx. 80 mg with the use of the Waters auto-purification system (description - see analytic methods).Montelukast diastereoisomer I:1H NMR (500 MHz, CDCl3), δ (ppm): 0.38 (m, 2H) ; 0.41-0.48 (m, 4H); 0.56(m, 2H); 1.60(s, 3H); 1.61(s, 3H); 2.00(d, IH); 2.40(d, IH); 2.16(q,2H); 2.33(d, IH); 2.43(d, IH); 2.35(d, IH); 2.55(d, IH); 2.37(d, IH); 2.49(d, IH); 2.97(q, 2H); 3.37(dd, IH); 3.62(dd, IH); 3.93(t, IH); 4.29(dd, IH); 7.1 l(m, IH); 7.17(m, 2H); 7.23(m, IH); 7.24(m, IH); 7.28(m, IH); 7.30(m, IH); 7.38(d, IH); 7.4(dd, lH);7.70(d, IH); 8.08(d, IH); 8.1 l(d, IH).
References:
WO2009/111998,2009,A2 Location in patent:Page/Page column 25-26
![METHYL 2-(3-{(E)-3-[2-(7-CHLORO-2-QUINOLYL)VINYL]PHENYL}-3-OXOPROPYL)BENZOATE](/CAS/GIF/149968-11-6.gif)
149968-11-6
96 suppliers
$120.09/10mg

1187586-61-3
71 suppliers
inquiry

1187586-58-8
63 suppliers
inquiry
![METHYL 2-[(S)-3-{(E)-3-[2-(7-CHLORO-2-QUINOLYL)VINYL]PHENYL}-3-HYDROXYPROPYL]BENZOATE](/CAS/GIF/181139-72-0.gif)
181139-72-0
56 suppliers
$401.75/1g

1187586-61-3
71 suppliers
inquiry

1187586-58-8
63 suppliers
inquiry
![2-[3-(S)-[3-(2-(7-Chloro-2-quinolinyl)ethenyl)phenyl]-3-hydroxypropyl]phenyl-2-propanol](/CAS2/GIF/287930-77-2.gif)
287930-77-2
63 suppliers
inquiry

1187586-61-3
71 suppliers
inquiry

1187586-58-8
63 suppliers
inquiry
918972-54-0
85 suppliers
$125.00/5mg

1187586-61-3
71 suppliers
inquiry

1187586-58-8
63 suppliers
inquiry