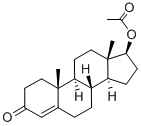
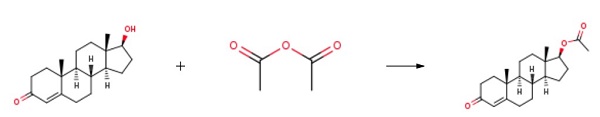
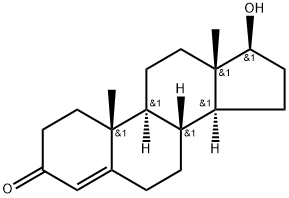
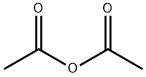
Testosterone acetate synthesis
- Product Name:Testosterone acetate
- CAS Number:1045-69-8
- Molecular formula:C21H30O3
- Molecular Weight:330.46
58-22-0
410 suppliers
$21.00/1mg
108-24-7
5 suppliers
$14.00/250ML

1045-69-8
235 suppliers
$28.00/1mg
Yield:1045-69-8 100%
Reaction Conditions:
with pyridine;dmap at 20; for 24 h;Inert atmosphere;
Steps:
4.1. Testosterone-17β-acetate (S1)
Testosterone 4 (500 mg, 1.73 mmol) was dissolved in 2.5 mLacetic anhydride. Then, 4 mg (0.035 mmol) of DMAP and 0.25 mLof dry pyridine were added. The mixture was stirred at room temperaturefor 24 h. The reaction was quenched with water and themixture was extracted with ethyl acetate (3 30 mL). The organiclayers were dried (Na2SO4) and concentrated under reduced pressure.The crude product was used to the next step without furtherpurification (567 mg, 100% yield). The NMR spectral data wereaccordance with those reported in the literature [33]. S1: 1HNMR (500 MHz, CDCl3) d 5.73 (s, 1H), 4.60 (dd, J = 9.0, 8.0 Hz,1H), 2.48-2.25 (m, 4H), 2.18 (m, 1H), 2.04 (s, 3H), 2.03-1.98 (m,1H), 1.77-1.87 (m, 2H), 1.75-1.62 (m, 2H), 1.61-1.47 (m, 3H),1.46-1.26 (m, 2H), 1.21-1.16 (m, 1H), 1.19 (s, 3H), 1.05-0.9 (m,3H), 0.84 (s, 3H).
References:
Trafalis, Dimitrios;Geromichalou, Elena;Dalezis, Panagiotis;Nikoleousakos, Nikolaos;Sarli, Vasiliki [Steroids,2016,vol. 115,p. 1 - 8]
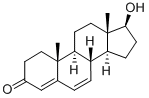
2352-19-4
8 suppliers
inquiry

1045-69-8
235 suppliers
$28.00/1mg

58-22-0
410 suppliers
$21.00/1mg
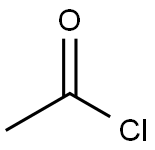
75-36-5
577 suppliers
$17.92/100G

1045-69-8
235 suppliers
$28.00/1mg
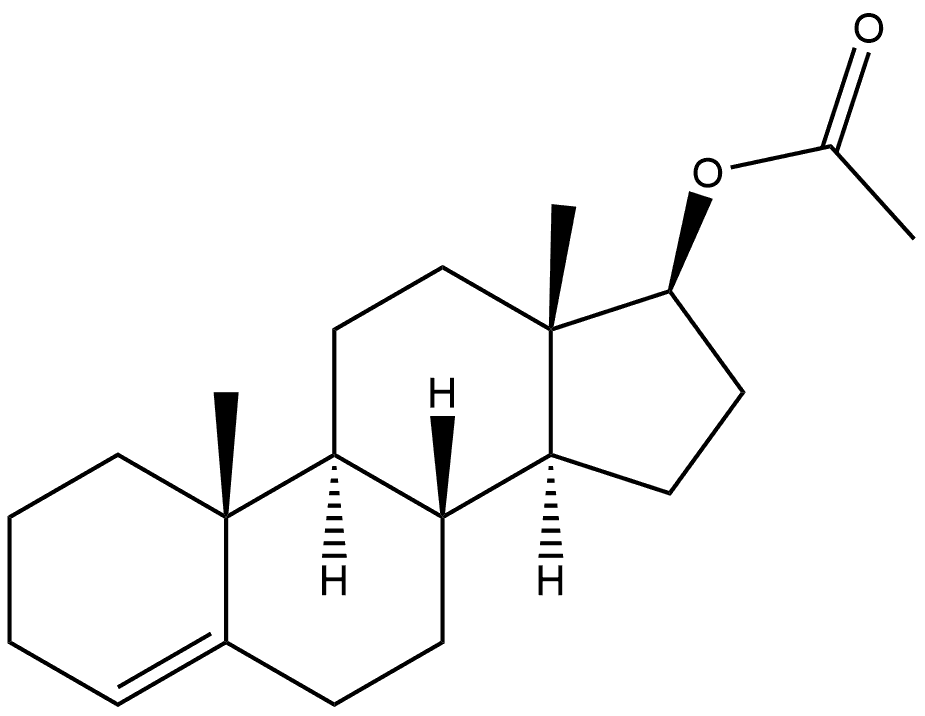
6157-79-5
0 suppliers
inquiry

1045-69-8
235 suppliers
$28.00/1mg
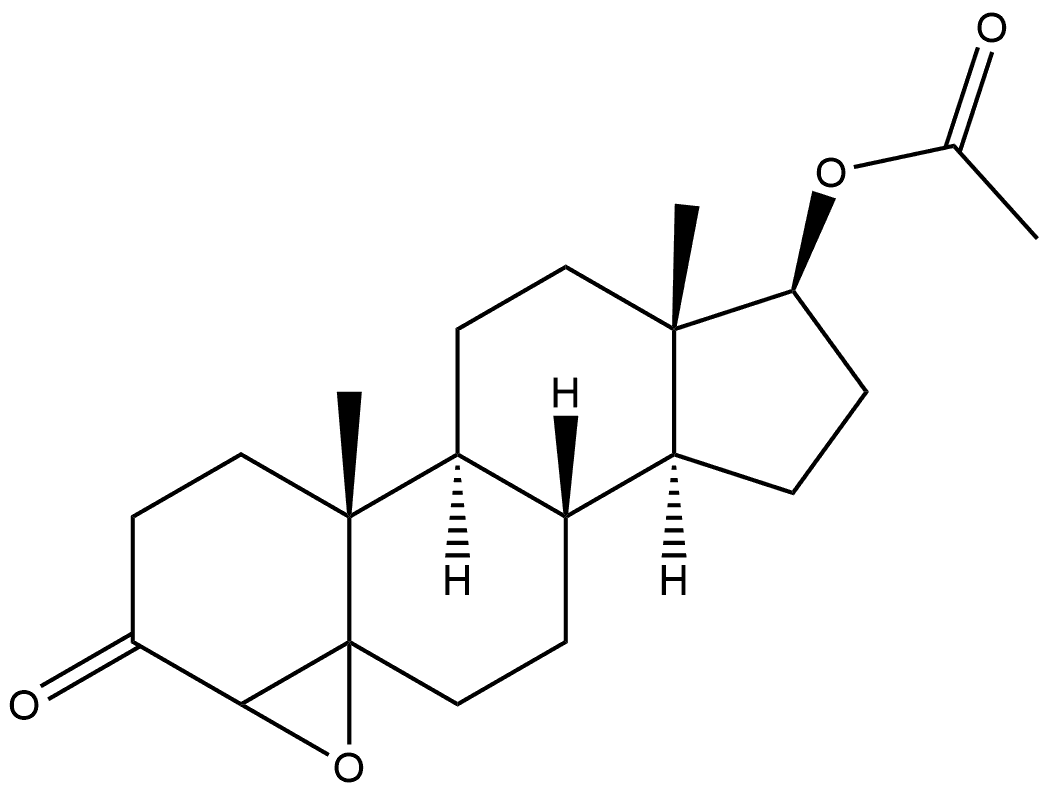
2944-75-4
0 suppliers
inquiry

1045-69-8
235 suppliers
$28.00/1mg