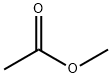
Methyl acetate synthesis
- Product Name:Methyl acetate
- CAS Number:79-20-9
- Molecular formula:C3H6O2
- Molecular Weight:74.08
2–1-Reactions
In the presence of strong bases such as sodium hydroxide or strong acids such as hydrochloric acid or sulfuric acid it is hydrolyzed back into methanol and acetic acid, especially at elevated temperature. The conversion of methyl acetate back into its components, by an acid , is a first-order reaction with respect to the ester. The reaction of methyl acetate and a base, for example sodium hydroxide, is a second-order reaction with respect to both reactants.
3-Applications
A major use of methyl acetate is as a volatile low toxicity solvent in glues, paints, and nail polish removers. Acetic anhydride is produced by carbonylation of methyl acetate in a process that was inspired by the Monsanto acetic acid synthesis.

67-56-1
762 suppliers
$9.00/25ml

108-21-4
600 suppliers
$10.00/25ml

79-20-9
560 suppliers
$14.00/100g
Yield:79-20-9 100%
Reaction Conditions:
with dilithium tetra(tert-butyl)zincate at 0; for 1 h;Inert atmosphere;
Steps:
General procedure: A typical procedure was as follows. Vinyl acetate (5.0mmol), benzyl alcohol (1.0mmol), and solvent (2.0mL) were added to a glass ampoule which had been degassed and filled with nitrogen. A solution of TBZL (0.1mmol) in THF was added under a nitrogen atmosphere to the ampoule placed in a constant-temperature bath. Then the mixture was kept at 25 or 0°C and stirred. After a prescribed period, the mixture was diluted with CDCl3 and transformed in a NMR tube. Conversion of the reaction was determined by 1H NMR at 400MHz.
References:
Oshimura, Miyuki;Oda, Yuki;Kondoh, Keita;Hirano, Tomohiro;Ute, Koichi [Tetrahedron Letters,2016,vol. 57,# 19,p. 2070 - 2073]

67-56-1
762 suppliers
$9.00/25ml
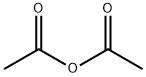
108-24-7
5 suppliers
$14.00/250ML

79-20-9
560 suppliers
$14.00/100g

67-56-1
762 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry

79-20-9
560 suppliers
$14.00/100g
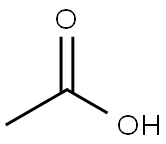
64-19-7
1568 suppliers
$10.00/25ML
![[methoxy(dimethyl)silyl]methyl acetate](/CAS/20200331/GIF/18162-90-8.gif)