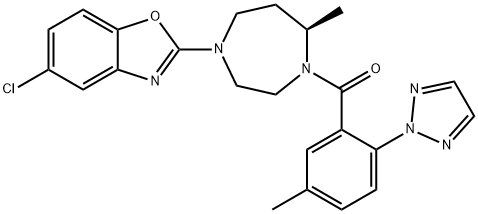
Suvorexant synthesis
- Product Name:Suvorexant
- CAS Number:1030377-33-3
- Molecular formula:C23H23ClN6O2
- Molecular Weight:450.92
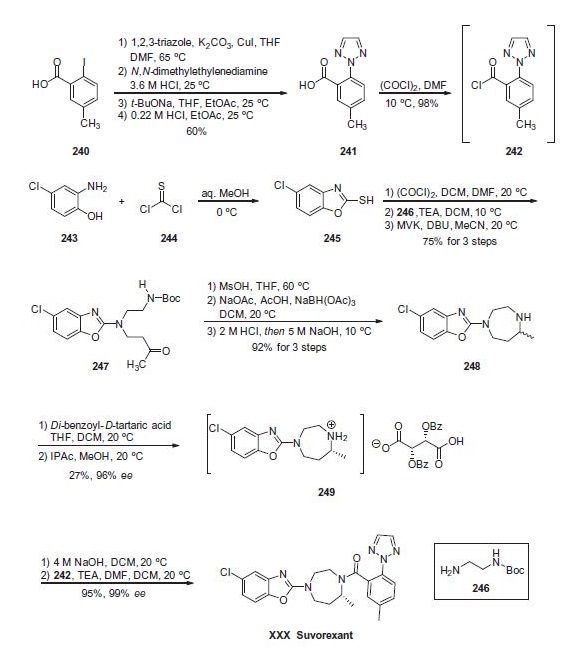
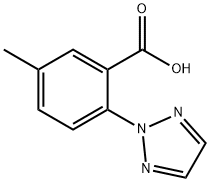
956317-36-5
224 suppliers
$7.00/250mg
![(R)-5-chloro-2-(5-Methyl-1,4-diazepan-1-yl)benzo[d]oxazole](/CAS/GIF/1266975-27-2.gif)
1266975-27-2
71 suppliers
$374.00/250mg

1030377-33-3
219 suppliers
$39.00/1mg
Yield:1030377-33-3 97%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in N,N-dimethyl-formamide at 0 - 20;Reagent/catalyst;Solvent;Temperature;
Steps:
1 Example 1:
Diazepane intermediate I (75 mmol) was dissolved in N, N-dimethylformamide (75 ml) followed by reducing body Department of temperature to 0 ~ 5 , was added triazole intermediates II (79mmol),Hydroxybenzotriazole (82.8 mmol), EDCI (82.8 mmol) and triethylamine (188 mmol) were added and the mixture was warmed naturally and stirred at room temperature overnight. Adding 10% citric acid solution, extracting with ethyl acetate, washing the organic layer with 5% sodium carbonate solution, washing with saturated saline solution, drying with anhydrous sodium sulfate, filtering and concentrating to obtain 32.8g of suvresol, yield 97%, Purity: 98.1%; the resulting product was further treated with isopropyl acetate and n-propyl acetate Recrystallization from heptane gave 31.8 g of a white solid in 94% yield
References:
CN106916149,2017,A Location in patent:Paragraph 0018; 0019; 0020; 0021; 0022; 0023; 0024-0045
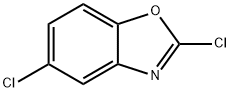
3621-81-6
168 suppliers
$8.00/1g
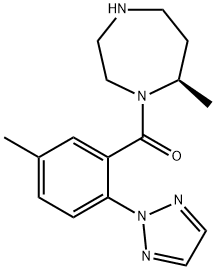
1030377-32-2
24 suppliers
inquiry

1030377-33-3
219 suppliers
$39.00/1mg

1030377-32-2
24 suppliers
inquiry
17200-29-2
128 suppliers
$5.00/250mg

1030377-33-3
219 suppliers
$39.00/1mg

956317-36-5
224 suppliers
$7.00/250mg
![5-Chloro-2-((R)-5-Methyl-[1,4]diazepan-1-yl)benzooxazole hydrochloride](/CAS/20150408/GIF/1266664-66-7.gif)
1266664-66-7
77 suppliers
inquiry

1030377-33-3
219 suppliers
$39.00/1mg

1030377-32-2
24 suppliers
inquiry
![2-Bromo-5-chlorobenzo[d]oxazole](/CAS/20150408/GIF/CB02503637.gif)
1251033-26-7
8 suppliers
inquiry

1030377-33-3
219 suppliers
$39.00/1mg