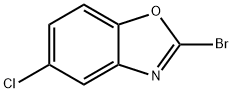
2-Bromo-5-chlorobenzo[d]oxazole synthesis
- Product Name:2-Bromo-5-chlorobenzo[d]oxazole
- CAS Number:1251033-26-7
- Molecular formula:C7H3BrClNO
- Molecular Weight:232.46
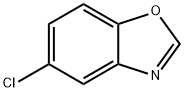
17200-29-2
131 suppliers
$5.00/250mg
![2-Bromo-5-chlorobenzo[d]oxazole](/CAS/20150408/GIF/CB02503637.gif)
1251033-26-7
8 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 5-chloro-benzoxazolewith lithium hexamethyldisilazane in tetrahydrofuran at -25 - -15; for 2.83333 h;Inert atmosphere;
Stage #2: with N-Bromosuccinimide in tetrahydrofuran at -25 - 20; for 3.33333 h;Inert atmosphere;
Steps:
Experimental
General procedure: A 500 mL 3-neck round bottom flask equipped with a septum, thermocouple, 125 mL addition funnel, inert gas inlet and magnetic stir bar was purged with nitrogen for 10 min. Hexamethyldisilazane (42 mL, 0.20 mol) and THF (78 mL) were charged against positive nitrogen pressure. The addition funnel was charged with a hexane solution of n-butyllithium (78.0 mL, 195 mmol). The amine solution was cooled to -52 °C and n-butyllithium was added over 84 min, resulting in a temperature increase to 12.5 °C over the course of the addition. The resulting lithium hexamethyldisilazide solution was removed from the cooling bath and aged for 30 min.To a 500 mL 3-neck round bottom flask equipped with a septum, thermocouple, inert gas inlet and magnetic stir bar was charged 5-chlorobenzoxazole (20.00 g, 130 mmol). The gray solid was dissolved in THF (100 mL) and the resulting colorless solution was cooled to -25 °C. The freshly prepared lithium hexamethyldisilazide solution was added via cannula over 80 min. The temperature of the anion solution was maintained between -25 and -15 °C during the addition. The resulting dark brown solution was aged for 90 min between -25 and -15 °C.To a 1000 mL 3-neck round bottom flask equipped with a Claisen adapter, septum, thermocouple, inert gas inlet, stir shaft bearing, and blade was charged THF (100 mL) and N-bromosuccinimide (34.8 g, 195 mmol). The resulting slurry was cooled to -20 °C and the anion solution was added via cannula over 150 min. During the addition the anion solution and reaction mixture were maintained between -25 and -15 °C. The resulting brown slurry was removed from the cooling bath and aged for 50 min while warming to room temperature.To the resulting bromide slurry was added a solution of ethanolamine (12.6 mL, 208 mmol) in MeCN (38 mL) via syringe pump over 5 h. During the addition the reaction temperature was maintained between 20 and 27 °C. The resulting brown slurry was aged at room temperature overnight.The reaction mixture was cooled in an ice water bath and the septum replaced with a 50 mL addition funnel charged with concentrated HCl (32 mL, 390 mmol). The acid solution was added over 10 min, during which time the temperature increased from 10 to 20 °C. The reaction mixture was removed from the ice water bath and aged for 5 min. Charged 20 wt % aqueous K2HPO4 (170 mL) and the resulting biphasic mixture was transferred to a separatory funnel. The flask was washed with THF (3×, 10 mL) and the washings were added. The aqueous phase was cut. The organic phase was washed with 20 wt % aqueous K2HPO4 (200 mL), separated and analyzed. The crude reaction stream had a total mass of 396.47 g. By quantitative HPLC assayed 25.81 g of 2 in the organic phase, 93% assay yield, 92.74 LC peak area percent at 210 nm. Pure 2 was obtained on a small (1 g) scale by silica gel chromatography (3:1 to 1:3 hexanes/EtOAc eluent). 1H NMR (500 MHz, DMSO-d6): δ = 8.17 (t, J = 5.6 Hz, 1H), 7.34 (d, J = 8.4 Hz, 1H), 7.25 (d, J = 1.8 Hz, 1H), 6.97 (dd, J = 8.4, 1.8 Hz, 1H), 4.81 (t, J = 5.4 Hz, 1H), 3.56 (q, J = 5.7 Hz, 2H), 3.35 ppm (q, J = 5.8 Hz, 2H). 13C NMR (100 MHz, DMSO-d6): δ = 163.5, 146.8, 145.0, 127.7, 119.5, 115.0, 109.4, 59.3, 45.0 ppm.
References:
Sherry, Benjamin D.;Chen, Yeo-Chuin Justin;Mangion, Ian K.;Yin, Jingjun [Tetrahedron Letters,2012,vol. 53,# 7,p. 730 - 732] Location in patent:experimental part
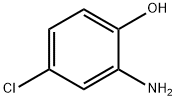
95-85-2
401 suppliers
$13.00/25g
![2-Bromo-5-chlorobenzo[d]oxazole](/CAS/20150408/GIF/CB02503637.gif)
1251033-26-7
8 suppliers
inquiry