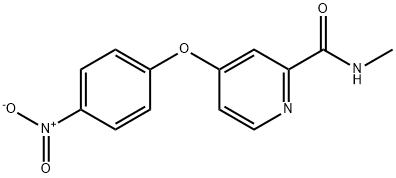
Sorafenib Impurity 35 synthesis
- Product Name:Sorafenib Impurity 35
- CAS Number:864272-34-4
- Molecular formula:C13H11N3O4
- Molecular Weight:273.24
Yield:864272-34-4 88.2%
Reaction Conditions:
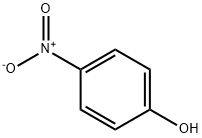
Stage #1: 4-nitro-phenolwith sodium hydroxide in water;isopropyl alcohol at 25; for 1 h;
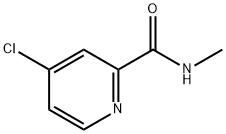
Stage #2: 4-chloro-N-methylpicolinamidewith tetrabutylammomium bromide;potassium carbonate in water;isopropyl alcohol at 70 - 80;Reagent/catalyst;
Steps:
1.1
Step 1: Take 16.69 g (0.12 mol) of p-nitrophenol into a three-necked flask, add 200 ml of isopropanol and 20 ml of water.Turn on the stirring, control the temperature below 25 ° C, and add 4.8 g (0.12 mol) of sodium hydroxide.The temperature was controlled below 25 ° C and stirred at 25 ° C for 1 h.Add 13.9 g (0.1 mol) of potassium carbonate and 0.5 g of tetrabutylammonium bromide.N-methyl(4-chloro-2pyridyl)carboxamide 17.06 g (0.1 mol),Stir well and warm to 70-80 ° C.TLC traces the disappearance of N-methyl(4-chloro-2pyridyl)formamide starting material.After the reaction was completed, it was cooled to room temperature, 300 g of water was added, and the mixture was stirred uniformly, and 300 g*2 was extracted with ethyl acetate.The organic phases were combined and washed once with 300 g of 10% sodium hydroxide and 300 g of 10% aqueous sodium chloride solution.The aqueous phase was separated and the organic phase was dried with 100 g of anhydrous sodium sulfate.Filtered, and the filtrate was concentrated under reduced pressure.An oily product of 24.1 g was obtained in a yield of 88.2%.
References:
CN105085388,2018,B Location in patent:Paragraph 0028; 0031; 0032; 0035; 0038; 0041; 0044; 0047
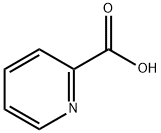
98-98-6
618 suppliers
$5.00/10g

864272-34-4
17 suppliers
inquiry
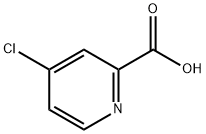
5470-22-4
312 suppliers
$7.00/5g

864272-34-4
17 suppliers
inquiry
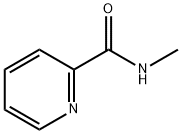
6144-78-1
35 suppliers
$135.00/500mg

864272-34-4
17 suppliers
inquiry