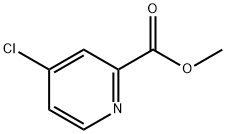
Methyl 4-chloropicolinate synthesis
- Product Name:Methyl 4-chloropicolinate
- CAS Number:24484-93-3
- Molecular formula:C7H6ClNO2
- Molecular Weight:171.58
Yield:24484-93-3 85%
Reaction Conditions:
Stage #1:2-Picolinic acid with thionyl chloride;N,N-dimethyl-formamide at 20 - 72; for 16.6667 h;
Stage #2:methanol at 20 - 55; for 0.75 h;
Stage #3: with water;sodium hydrogencarbonate in methanol; pH=8 - 9 at 45;
Steps:
1.a
Example 1Preparation of (R)-4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)-N-(1-(hydroxyamino)-1-oxopropan-2-yl)picolinamide (Compound 1)Step 1a. Methyl 4-chloropicolinate (Compound 102); Anhydrous DMF (10 mL) was slowly added to SOCl2 (300 mL) at 40-48° C. The solution was stirred at room temperature for 10 minutes, and then compound 101 (100.0 g, 813.0 mmol) was added over 30 minutes. The resulting solution was heated at 72° C. (Vigorous SO2 evolution) for 16 h to generate a yellow solid. The resting mixture was cooled to room temperature, diluted with toluene (500 mL) and concentrated to 200 mL. The toluene addition/concentration process was repeated twice. The resulting solution and solid was added into 200 mL methanol at ice bath to keep the internal temperature below 55° C. The content were stirred at r.t. for 45 min, cooled to 5° C. and treated with Et2O (200 mL) dropwise. The resulting solid were filtered, washed with Et2O (200 mL) and dried under 35° C. to provide a white yellow solid. After the solid were solvated to hot water (500 mL, about 45° C.), NaHCO3 was added to adjust pH to 8-9. The mixture was extracted with ethyl acetate and the organic phase was concentrated to give desired compound 102 as a off-white solid (118.2 g, 85%). LCMS: 172 [M+1]+.
References:
Cai, Xiong;Qian, Changgeng;Gould, Stephen;Zhai, Haixiao US2008/234332, 2008, A1 Location in patent:Page/Page column 22; 25

67-56-1
775 suppliers
$7.29/5ml-f
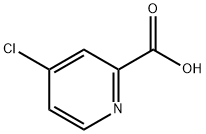
5470-22-4
316 suppliers
$7.00/5g

24484-93-3
411 suppliers
$6.00/5g

67-56-1
775 suppliers
$7.29/5ml-f

24484-93-3
411 suppliers
$6.00/5g
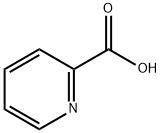
98-98-6
631 suppliers
$5.00/10g

24484-93-3
411 suppliers
$6.00/5g

5470-22-4
316 suppliers
$7.00/5g
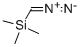
18107-18-1
215 suppliers
$33.00/5g

24484-93-3
411 suppliers
$6.00/5g