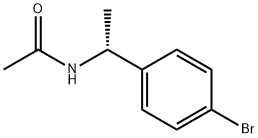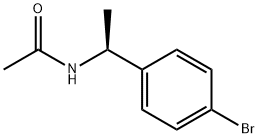
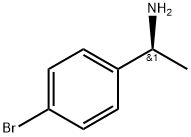
(S)-N-(1-(4-bromophenyl)ethyl)acetamide synthesis
- Product Name:(S)-N-(1-(4-bromophenyl)ethyl)acetamide
- CAS Number:186296-23-1
- Molecular formula:C10H12BrNO
- Molecular Weight:242.11
177750-12-8
10 suppliers
inquiry

186296-23-1
15 suppliers
inquiry
Yield:186296-23-1 100%
Reaction Conditions:
with bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate;C46H34O6P2;hydrogen in dichloromethane at 25; for 6 h;Autoclave;Inert atmosphere;enantioselective reaction;
Steps:
2.4 Representative Procedure for the Rh-CatalyzedAsymmetric Hydrogenation
General procedure: After stirring a solution of [Rh(cod)2]BF4 (0.0025 mmol)and ligand 2a (0.00275 mmol) in degassed CH2Cl2(1 mL)at room temperature for 1 h, a solution of the correspondingsubstrate (0.25 mmol) in degassed CH2Cl2(2 mL) wasadded. The mixture was transferred via syringe into a stainlessautoclave that had been previously purged with argon.The hydrogenation was carried out in the autoclave at roomtemperature for 6 h. After releasing H2,the reaction mixturewas passed through a short silica gel column to removethe catalyst. After evaporation of the solvent under reduced pressure, the residue was purified by flash chromatographyon silica gel. The data on conversion and enantiomericexcess of the product were determined by GC witha gamma-DEX 225 column (30 m × 0.25 mm × 0.25 μmfilm thickness) CP-Chirasil-DEX CB column(25 m × 0.25 mm × 0.25 μm film thickness) or AT FFAP(30 m × 0.25 mm × 0.25 μm film thickness). The absoluteconfiguration was determined by comparison with authenticsamples [37].
References:
Pang, Zengbo;Tian, Mi;Li, Haifeng;Wang, Lailai [Catalysis Letters,2017,vol. 147,# 4,p. 893 - 899]
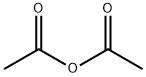
108-24-7
5 suppliers
$14.00/250ML
27298-97-1
258 suppliers
$9.00/1g

186296-23-1
15 suppliers
inquiry

27298-97-1
258 suppliers
$9.00/1g
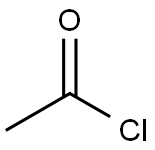
75-36-5
578 suppliers
$17.92/100G

186296-23-1
15 suppliers
inquiry