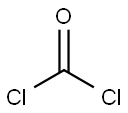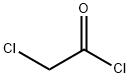
Chloroacetyl chloride synthesis
- Product Name:Chloroacetyl chloride
- CAS Number:79-04-9
- Molecular formula:C2H2Cl2O
- Molecular Weight:112.94
Koenig G et al; Chloroacetic Acids. Ullmann's Encyclopedia of Industrial Chemistry 7th ed. (1999-2018). NY, NY: John Wiley & Sons. Online Posting Date: October 15, 2012
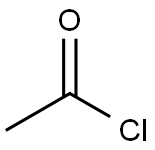
75-36-5
577 suppliers
$17.92/100G

79-04-9
362 suppliers
$12.00/5g
Yield:79-04-9 89.59%
Reaction Conditions:
with chlorine at 55; under 750.075 - 1500.15 Torr;Temperature;
Steps:
4-6 Example 6
Controlling the gaseous flow rate allows the gaseous acetyl chloride and chlorine gas to simultaneously enter the contact reactor at a molar ratio of 1:1.2 at a pressure of 0.1 to 0.2 MPa, and uniformly fill the 5-layer flow with a catalyst containing 7% by mass of the total acetyl chloride of the reactant. In the chemical bed, the activated carbon supported on the activated carbon prepared in Example 2 was used as the reaction catalyst, and the reaction temperature was maintained at 55±2 ° C. The chloroacetyl chloride formed by the reaction was transferred to the product tank through the bottom of the reactor in time, and the hydrogen chloride gas formed by the reaction was The condenser connected to the top of the reactor enters the hydrogen chloride gas falling film absorption tower, and the condensed small amount of acetyl chloride is recycled by gasification, and the separated chlorine gas is dried by concentrated sulfuric acid and then recycled to the reactor for recycling. The yield was 89.59%, and the colorless transparent liquid chloroacetyl chloride was obtained, and the purity of the detection chromatogram was 99.18%.
References:
CN109456168,2019,A Location in patent:Paragraph 0020-0022
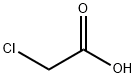
79-11-8
10 suppliers
$27.00/25g

79-04-9
362 suppliers
$12.00/5g

75-36-5
577 suppliers
$17.92/100G
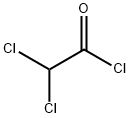
79-36-7
290 suppliers
$15.00/10g

79-04-9
362 suppliers
$12.00/5g
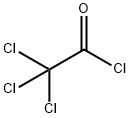
76-02-8
248 suppliers
$38.29/25G
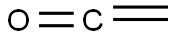
463-51-4
15 suppliers
inquiry

79-04-9
362 suppliers
$12.00/5g