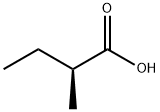
(S)-(+)-2-Methylbutyric acid synthesis
- Product Name:(S)-(+)-2-Methylbutyric acid
- CAS Number:1730-91-2
- Molecular formula:C5H10O2
- Molecular Weight:102.13
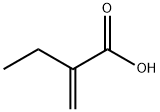
3586-58-1
31 suppliers
$275.00/250mg
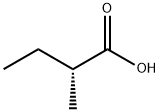
32231-50-8
67 suppliers
$79.00/100mg

1730-91-2
109 suppliers
$32.00/100mg
Yield: 2 % ee
Reaction Conditions:
with 5%-palladium/activated carbon;hydrogen;benzylamine;Cinchonidin in 1,4-dioxane;water at 23; under 37503.8 Torr; for 5 h;Autoclave;enantioselective reaction;
Steps:
2. Experimental
A 5% Pd/C catalyst was obtained from N.E.Chemcat (5%STD type) in a 51% wet form. The 5% Pd/C catalyst (20 mg)was pretreated at 80 °C in 2.5% (v/v) aqueous dioxane (5 mL)under atmospheric hydrogen for 30min. After cooling to 23 °C,a solution of cinchonidine (12 mg, 0.04mmol) in the aqueousdioxane (1 mL) was added to the catalyst, and the mixture wasstirred further for 30min. A solution of 1 (50 mg, 0.5mmol)and benzylamine (0.5mmol) in the aqueous dioxane (4 mL)was added to the cinchonidine-modified catalyst while stirring.After the substrate was fully converted to the expected productas measured by hydrogen consumption, the reaction mixturewas quenched with HCl aqueous solution and filtered. Thefiltrate was extracted with ether, and analyzed by 1HNMR,ESI-MS, and GC. Enantiomeric ratio of 3 was determined bygas chromatography equipped with a chiral column (Chirasil-DEX-CB, 25m 0.25mm 0.25 m film thickness). Retentiontimes of (S)-3 and (R)-3 were 17.8 and 19.2min, respectively,when the column temperature was maintained at 95 °C.Initial rates for the hydrogenation were calculated from thehydrogen-uptake up to 25% conversion.Hydrogenation under 5 MPa of H2 was carried out using areactor made of stainless steel (SUS 316). The mixed suspensioncontaining 1 and CD-modified Pd/C was prepared by thesame procedures described above, and poured into the metalreactor. Hydrogen was introduced from an inlet of the reactor,and the reaction was carried out at 23 °C for 5 h while stirring
References:
Sugimura, Takashi;Tomatsuri, Satoshi;Fujita, Morifumi;Okamoto, Yasuaki [Bulletin of the Chemical Society of Japan,2019,vol. 92,# 10,p. 1737 - 1742]
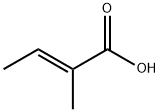
80-59-1
311 suppliers
$5.00/250mg

1730-91-2
109 suppliers
$32.00/100mg

3586-58-1
31 suppliers
$275.00/250mg

1730-91-2
109 suppliers
$32.00/100mg
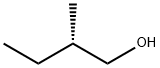
1565-80-6
134 suppliers
$6.00/5g

1730-91-2
109 suppliers
$32.00/100mg

80-59-1
311 suppliers
$5.00/250mg

32231-50-8
67 suppliers
$79.00/100mg

1730-91-2
109 suppliers
$32.00/100mg