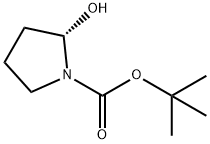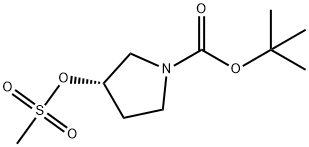
(S)-1-Boc-3-methanesulfonyloxy-pyrrolidine synthesis
- Product Name:(S)-1-Boc-3-methanesulfonyloxy-pyrrolidine
- CAS Number:132945-75-6
- Molecular formula:C10H19NO5S
- Molecular Weight:265.33

24424-99-5
834 suppliers
$13.50/25G
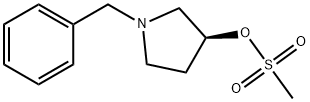
118354-71-5
20 suppliers
inquiry

132945-75-6
47 suppliers
$9.00/250mg
Yield:132945-75-6 96%
Reaction Conditions:
5%-palladium/activated carbon in methanol at 24; for 3 h;Product distribution / selectivity;
Steps:
1
Production Example 1: Production of (S)-1-(tert-butoxycarbonyl)-3-(methanesulfonyloxy)pyrrolidine; (S)-1-benzyl-3-(methanesulfonyloxy)pyrrolidine produced by the method described in Reference Example 1, in an amount of 1.39 g, was dissolved in 10 ml of methanol, and thereto were added 1.13 g of di t-butyl dicarbonate and 127 mg of 5% by weight palladium-carbon. Then the mixture was stirred in a hydrogen atmosphere under an ordinary pressure at 24°C for 3 hrs. After palladium was filtrated under reduced pressure, washing obtained using 30 ml of methanol was included. Thus obtained filtrate was concentrated under reduced pressure to give 1.37 g of the title compound as a pale yellow oily material (chemical purity: 90.9 area%, yield: 96%). The title compound could be obtained at an extremely high yield as compared with the case of Comparative Production Example 1.
References:
Kaneka Corporation EP2050735, 2009, A1 Location in patent:Page/Page column 19
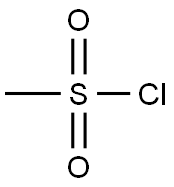
124-63-0
6 suppliers
$10.00/5g
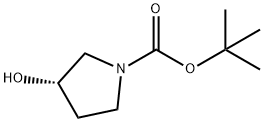
101469-92-5
354 suppliers
$6.00/5g

132945-75-6
47 suppliers
$9.00/250mg
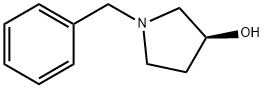
101385-90-4
302 suppliers
$12.00/1g

132945-75-6
47 suppliers
$9.00/250mg

24424-99-5
834 suppliers
$13.50/25G

132945-75-6
47 suppliers
$9.00/250mg