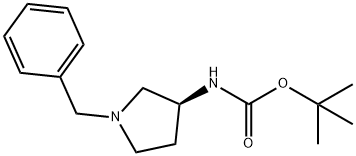
(S)-(-)-1-BENZYL-3-(BOC-AMINO)PYRROLIDINE synthesis
- Product Name:(S)-(-)-1-BENZYL-3-(BOC-AMINO)PYRROLIDINE
- CAS Number:131852-53-4
- Molecular formula:C16H24N2O2
- Molecular Weight:276.37

24424-99-5
833 suppliers
$13.50/25G
114715-38-7
193 suppliers
$9.00/1g

131852-53-4
71 suppliers
$20.00/5g
Yield:131852-53-4 94.1%
Reaction Conditions:
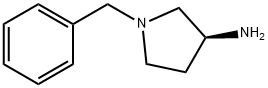
Stage #1:(3S)-1-benzyl-3-pyrrolidinamine with sodium hydroxide in water; pH=11 at 50 - 60;
Stage #2:di-tert-butyl dicarbonate in water; pH=11 for 3 h;
Steps:
3
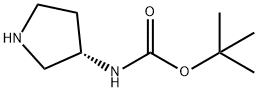
A 500 ml four-neck flask equipped with a stirrer, a thermometer, a Dimroth condenser, and a titration funnel was loaded with (S)-3-amino-1-benzylpyrrolidine 17.6 g (0.1 mole, optical purity 99.5%/ee), water 158.7 g, and Cation DS (manufactured by Sanyo Chemical Industries, Ltd.) 0.2 g and the pH of the mixture was adjusted to be 11+/-0.5 with an aqueous 48% sodium hydroxide solution. While the mixture being stirred at 50 to 60°C, di-tert-butyl dicarbonate (hereinafter abbreviated as DiBoc) 26.2 g (0.12 mole) was dropwise added for about 2 hours. During the time, the reaction solution was adjusted at pH 11+/-0.5 with an aqueous 48% sodium hydroxide solution. After being stirred for further 1 hour, the reaction solution was cooled to a room temperature and precipitated crystal was separated by filtration. The crystal was vacuum dried at 50°C to obtain (S)-1-benzyl-3-tert-butoxycarbonylaminopyrrolidine 26.0 g. The yield was 94.1% and the chemical purity was 99.1 % and an optical purity was 99.5%ee. The same apparatus as that of Example 1 was loaded with (S)-1-benzyl-3-tert-butoxycarbonylaminopyrrolidine 26.0 g (optical purity 99.5%ee), water 120 g, and 5% Pd/C 2.6 g (PE type, 55.27% water content, manufactured by N. E. Chemcat Corp.) and the contents were stirred at reaction temperature of 40°C for 10 hours under hydrogen ventilation. When the reaction solution was analyzed by GC, and the peak of the raw material disappeared and other than toluene only 3-tert-butoxycarbonylaminopyrrolidine was detected. After completion of the reaction, Pd/C was removed by filtration and the filtrate was concentrated to 30 g by an evaporator. Next, the concentrated product was mixed with toluene and concentrated to 20 g to remove water by azeotropic boiling. While being mixed, the concentrated solution was mixed slowly with n-hexane 25 g to precipitate a crystal and further stirred for 2 hour in an ice bath. The precipitated crystal was separated by filtration and vacuum dried to obtain (S)-3-tert-butoxycarbonylaminopyrrolidine 15.4 g. The yield was 87.4%, the chemical purity was 99.5 area%, and an optical purity was 99.5 %ee. The water content was 0.4%.
References:
Toray Fine Chemicals Co., Ltd. EP1640364, 2006, A1 Location in patent:Page/Page column 10-11
100-52-7
947 suppliers
$17.30/2g
122536-76-9
291 suppliers
$7.00/1g

131852-53-4
71 suppliers
$20.00/5g
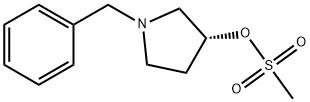
114715-35-4
6 suppliers
inquiry

131852-53-4
71 suppliers
$20.00/5g
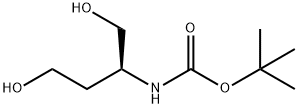
128427-10-1
157 suppliers
$18.00/250mg

131852-53-4
71 suppliers
$20.00/5g
![Carbamic acid, N-[(1S)-3-[(methylsulfonyl)oxy]-1-[[(methylsulfonyl)oxy]methyl]propyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/176970-13-1.gif)