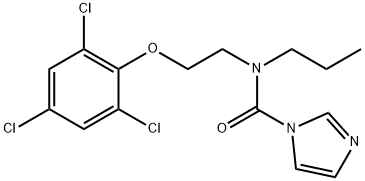
Prochloraz synthesis
- Product Name:Prochloraz
- CAS Number:67747-09-5
- Molecular formula:C15H16Cl3N3O2
- Molecular Weight:376.67
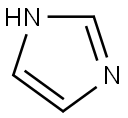
288-32-4
1010 suppliers
$5.00/25g
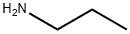
107-10-8
358 suppliers
$10.00/20mg
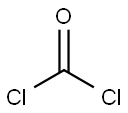
75-44-5
2 suppliers
inquiry

106-93-4
393 suppliers
$15.00/5g
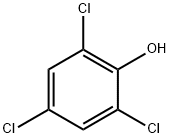
88-06-2
282 suppliers
$10.00/10g

67747-09-5
257 suppliers
$25.00/100mg
Yield:-
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;potassium carbonate in water;toluene
Steps:
1.2 EXAMPLE 1
(2) The 1-[N-propyl-N-2-(2,4,6-trichlorophenoxy)ethylcarbamoyl]imidazole employed in the above reaction was prepared in the following manner. A mixture of 197.5 g 2,4,6-trichlorophenol, 107.8 ml 1,2-dibromoethane and 500 ml water were stirred under reflux whilst sodium hydroxide solution (63 ml caustic soda liquor, specific gravity 1.5, and 61 ml water) was added slowly. The reaction mixture was heated under reflux for 16 hours and then the excess dibromoethane removed as an azeotrope, initially at atmospheric pressure and then under reduced pressure. To this mixture was then added 85 ml toluene and the temperature was maintained at about 50° C. The organic layer was separated and washed with dilute aqueous sodium hydroxide. To this organic layer was added 85 ml toluene, 137 ml sodium hydroxide (specific gravity 1.5), 137 ml water and 134 ml propylamine. The reagents were stirred at 50° to 60° C. for eight hours and then left at room temperature for 60 hours. The aqueous layer was separated and the organic layer was distilled at atmospheric pressure and then under reduced pressure. The distillate was slowly added to 39.7 g phosgene dissolved in 370 ml ice-cold toluene, keeping the temperature below 15° C. The mixture was then warmed to 70° C. and more phosgene passed in until a clear solution was formed. Toluene was removed under reduced pressure and the remaining solution cooled in ice. The solid formed was filtered off. This toluene solution was diluted with a further quantity of 250 ml toluene and stirred at 80° C. for two hours with 47.3 g imidazole and 96.0 g anhydrous potassium carbonate. The mixture was allowed to cool and 386 ml water added. The organic layer was separated, washed with water and then dried over anhydrous magnesium sulphate. This was then concentrated under reduced pressure, stirred with dilute hydrochloric acid and the aqueous layer adjusted to pH 8 and ether extracted to give 1-[N-propyl-N-2-(2,4,6-trichlorophenoxy)ethylcarbamoyl]imidazole. The following compounds were made by a similar method employing the appropriate imidazole compound and metal salt as reactants. bis-[1-{N-2-(2,4-dichloro-6-methoxylphenoxy)ethyl-N-propylcarbamoyl}imidazole]manganese (II) chloride complex, melting point 132°-134° C. bis-[1-{N-butyl-N-2-(2,4-dichloro-6-methylphenoxy)ethylcarbamoyl}imidazole]copper (II) chloride complex, melting point 136°-138° C. bis-[1-{N-butyl-N-2-(4-chloro-2,6-dibromophenoxy)ethylcarbamoyl}imidazole]copper (II) chloride complex, melting point 168°-169° C. bis-[1-{N-propyl-N-2-(2,4,6-trichlorophenoxy)ethylcarbamoyl}imidazole]copper (II) bromide complex, melting point 151°-153° C. bis-[1-{N-2-(2-bromo-4-chlorophenoxy)ethyl-N-butylcarbamoyl}imidazole]copper (II) chloride complex, melting point 130°-132° C. bis-[1-{N-propyl-N-2-(2,4,6-trichlorophenoxy)ethylcarbamoyl}imidazole]copper (II) nitrate complex, melting point 124°-126° C. bis-[1-{N-2-(2,4-dichloro-6-methylphenoxy)ethyl-N-propylcarbamoyl}imidazole]copper (II) chloride complex melting point 130°-132° C. bis-[1-{N-2-(2,4-dichloro-6-methylphenoxy)ethyl-N-propylcarbamoyl}imidazole]nickel chloride complex, melting point 158°-160° C. bis-[1-{N-propyl-N-2-(2,4,6-trichlorophenoxy)ethylcarbamoyl}imidazole]iron (II) chloride complex, melting point 136°-138° C. bis-1-(N-2-phenoxyethyl-N-propylcarbamoyl)imidazole copper (II) chloride complex, melting point 125°-127° C. bis-[1-(N-2,4-dichlorobenzyl-N-isopropylcarbamoyl)imidazole]copper (II) chloride complex, melting point 78°-80° C.
References:
The Boots Company Limited US4250179, 1981, A