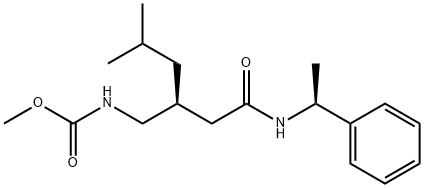
Pregabalin Impurity 12 synthesis
- Product Name:Pregabalin Impurity 12
- CAS Number:930280-44-7
- Molecular formula:C18H28N2O3
- Molecular Weight:320.43
Yield:930280-44-7 97%
Reaction Conditions:
with bromine;sodium methylate at -25 - 65; for 2 - 4 h;Product distribution / selectivity;Hofmann Rearrangement;
Steps:
7; 10; 13; 16; 20
three-necked flask equipped with an addition funnel, thermometer pocket, drying tube and a mechanical stirrer, was charged with methanol (2000 ml), (R)-3- isobutylpentanedioic acid amide-((S)-l-phenylmethyl) amide, compound of formula 1 (20Og, 0.689 mole) and cooled to 0° to 5°C followed by addition of sodium methoxide (149 g, 2.758 mole). The reaction mass was cooled to -15 to -25°C followed by addition of bromine (165.5 g, 1.034 mole) and stirred for 1-2 h at -15 to -25°C. The mixture was gradually warmed to a temperature of 0°C and then to 55-65°C, followed by stirring for 1 to 2 hours. The solvent was then stripped off and water was added to the mass. The resulted slurry was further extracted with toluene, toluene layer washed with brine followed by stripping off the solvent results in 215 g (97% yield) of {(S)-4-methyl-2-[((S)- 1 -phenylethylcarbamoyl)- methyl]pentyl}carbamic acid methyl ester (4) with a purity of 90.9% area, as measured by HPLC; Example 10: Preparation of (S)-PregabalinA three-necked flask equipped with an addition funnel, thermometer pocket, drying tube and a mechanical stirrer, was charged with methanol (2000 ml), (R)-3- isobutylpentanedioic acid amide-((S)-l-phenylmethyl) amide, compound of formula 1 (20Og, 0.689 mole) and cooled to 0° to 5°C followed by addition of sodium methoxide (149 g,2.758 mole). The reaction mass was cooled to -15 to -25°C followed by addition of bromine (165.5 g, 1.034 mole) and stirred for 1-2 h at -15 to -25°C. The mixture was gradually warmed to a temperature of 0°C and then to 55-65°C, followed by stirring for 1 to 2 hours. The solvent was then stripped off and water was added to the mass. The resulted slurry was further extracted with toluene, toluene layer washed with brine followed by stripping off the solvent. 4N hydrochloric acid (2580 ml), phenol (10.72 g, 0.114 mole) , sodium chloride (78.15 g, 1.342 mole) was added to the mass and was heated to 105°-l 100C for 15-24 hours, and then cooled to room temperature, i.e., about 20° to about 25°C. An aqueous 40% sodium hydroxide solution was added in an amount sufficient to provide a pH of 1. The solution was
References:
WO2008/118427,2008,A2 Location in patent:Page/Page column 16-17; 19; 21; 23
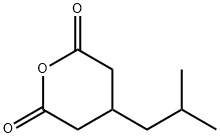
185815-59-2
118 suppliers
inquiry

930280-44-7
14 suppliers
inquiry
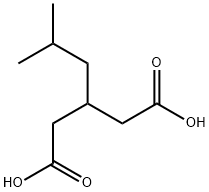
75143-89-4
278 suppliers
$7.00/10g

930280-44-7
14 suppliers
inquiry

![Pentanediamide, 3-(2-methylpropyl)-N1-[(1S)-1-phenylethyl]-, (3R)-](/CAS/20210305/GIF/930280-43-6.gif)
![Hexanoic acid, 5-methyl-3-[2-oxo-2-[[(1S)-1-phenylethyl]amino]ethyl]-, (3S)-](/CAS/20210305/GIF/930280-42-5.gif)