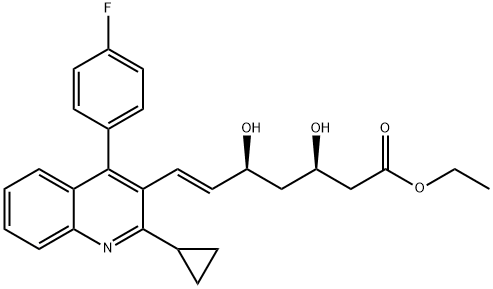
Pitavastatin Ethyl Ester synthesis
- Product Name:Pitavastatin Ethyl Ester
- CAS Number:167073-19-0
- Molecular formula:C27H28FNO4
- Molecular Weight:449.51
![6-Heptenoic acid, 7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-3,5-dioxo-, ethyl ester, (6E)-](/CAS/20210111/GIF/166803-31-2.gif)
166803-31-2
0 suppliers
inquiry

167073-19-0
53 suppliers
$742.00/250mg
Yield:100 % ee
Reaction Conditions:
with Candida famata var famata RIFY7455 in dimethyl sulfoxide at 30; pH=7; for 20 h;Product distribution / selectivity;aerobic conditions;potassium phosphate buffer (pH 7.0);
Steps:
1 Example 1; Production of DOLE from DOXE
Each kind of strains listed in Table 1 was inoculated in a liquid culture medium (2.5 mL) composed of 5 g/L of yeast extract (manufactured by Difco Co., Ltd.), 5 g/L of polypeptone (manufactured by Nihon Pharmaceutical Co., Ltd.), 3 g/L of malt extract (manufactured by Difco Co., Ltd.), and 20 g/L of glucose (manufactured by Nihon Shokuhinkako Co., Ltd.), and was then incubated at 30° C. for 21 hours under aerobic conditions. The resulting culture medium was centrifuged in an amount of 1 ml at a time to collect microbial cells. Then, 0.25 mL of a reaction solution containing the compound (I) (which is a compound, in the formula, R=ethyl group: DOXE) was added in the microbial cells to allow a reaction under aerobic conditions at 30° C. for 20 hours. [0133] The composition of the above reaction solution includes 0.3 g/L of DOXE, 20 g/L of glucose(manufactured by Nihon Shokuhinkako Co., Ltd.), 20 mL/L of dimethyl sulfoxide (DMSO) (manufactured by Kishida Chemical Co., Ltd.), and a 100 mM potassium phosphate buffer (pH 7.0). [0134] After terminating the reaction, 0.5 mL of ethyl acetate was added in the reaction solution and was mixed therewith vigorously, followed by separation into an organic layer and a water layer by a centrifugation. The organic layer was transferred to another container. A solvent was distilled off with a condensation centrifuge. Then, the dried solid product was dissolved in 0.01 mL of ethyl acetate, and was then subjected to a thin-layer chromatography (TLC). The TLC used a silica gel plate (silica gel 60 F254 manufactured by Merck & Co.), and developing solvent used was of hexane/ethyl acetate=1/1. [0135] After terminating the development, the product was confirmed with an UV lamp. As for the compound (I), Rf=0.76 to 0.86. As for compounds (II) and (III), Rf=0.54 to 0.61. As for the compound (IV) (which is a compound, in the formula, R=ethyl group: DOLE), Rf=0.33. A spot of the DOLE on the TLC was scraped and eluted with 0.25 mL of isopropanol. After the centrifugation, a supernatant was subjected to a high-performance liquid chromatography (HPLC) to analyze its optical purity and the concentration of a TLC-scraped-off sample. [0136] The following is the conditions of HPLC. [0137] Column: CHIRALCEL AD (manufactured by Daicel Chemical Industries, Ltd.) [0138] Eluting solution: Hexane/ethanol=9/1 [0139] Flow rate: 0.5 ml/min. [0140] Detection: UV 254 nm [0141] Temperature: Room temperature [0142] The results are listed in Table 1.
References:
US2004/30139,2004,A1 Location in patent:Page 12
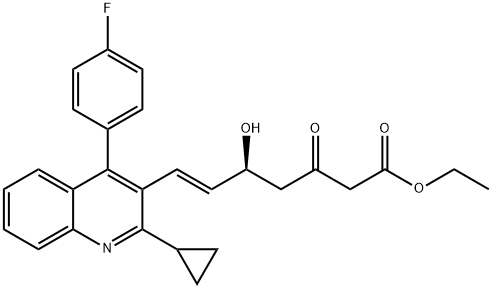
254452-91-0
9 suppliers
inquiry

167073-19-0
53 suppliers
$742.00/250mg
![6-Heptenoic acid, 7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-5-hydroxy-3-oxo-, ethyl ester, (5R,6E)-](/CAS/20210305/GIF/254452-95-4.gif)
254452-95-4
0 suppliers
inquiry

254452-91-0
9 suppliers
inquiry

167073-19-0
53 suppliers
$742.00/250mg
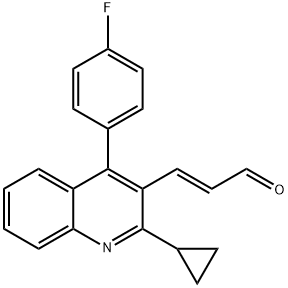
148901-68-2
110 suppliers
inquiry

167073-19-0
53 suppliers
$742.00/250mg
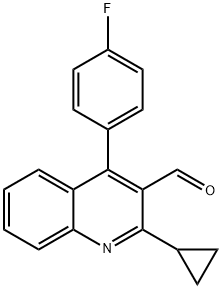
121660-37-5
180 suppliers
$9.00/250mg

167073-19-0
53 suppliers
$742.00/250mg