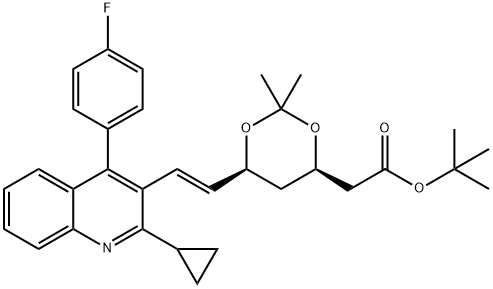
(4R,6S)-6-[(1E)-2-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester synthesis
- Product Name:(4R,6S)-6-[(1E)-2-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester
- CAS Number:147489-06-3
- Molecular formula:C32H36FNO4
- Molecular Weight:517.63
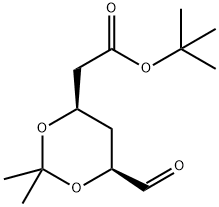
124752-23-4
238 suppliers
$9.00/250mg
![P-[[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]methyl]phosphonic acid diethyl ester](/CAS/GIF/154057-57-5.gif)
154057-57-5
11 suppliers
inquiry
![(4R,6S)-6-[(1E)-2-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester](/CAS/GIF/147489-06-3.gif)
147489-06-3
180 suppliers
$99.00/1g
Yield:147489-06-3 89%
Reaction Conditions:
with lithium chloride in tetrahydrofuran at 0 - 35; for 5.66667 h;Inert atmosphere;Reagent/catalyst;
Steps:
4; 6; 8; 10
35 g of compound 2, 210 ml of anhydrous THF, and 0.07 g of lithium chloride were placed in a reaction flask, nitrogen-protected, stirring was started, the temperature was lowered to 0-5 ° C, and 3.7 g of NaH (60%) was added.24 g of compound 3 ((4R-CIS)-6-formaldehyde-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester) was dissolved in 70 ml of anhydrous THF and added dropwise to the above reaction. In the liquid, add 40 minutes.The reaction was kept at 0-5 ° C for 1-2 hours, and the temperature was raised to 30-35 ° C for 5 hours.After TLC detection, the reaction was cooled to 0-5 ° C, and acetic acid was slowly added dropwise to pH = 7-7.5, and 70 ml of water was added. Static, liquid separation, collecting the upper organic phase. The organic phase is washed 1-2 times with water, dried over anhydrous sodium sulfate, and filtered to remove the desiccant.Distillation of THF under reduced pressure afforded 42 g of a yellow oil.The crude product yield was 95.8%. Add 95ml of isopropanol to the above crude product, start stirring, warm up to 75 ° C to dissolve, heat off for 30 minutes, start to cool down, cool down to 5 ° C for 3 hours, keep warm for 1 hour, suction filtration, filter cake with a small amount of ice isopropyl Alcohol wash, yield cake, dry,Obtained 39 g of compound B as a white solid.HPLC = 99.3% (E configuration), total yield 89%.
References:
Hangzhou Ledun Technology Co., Ltd.;Ren Fengbo;Yang Pingfeng;Zhou Yifeng CN108822090, 2018, A Location in patent:Paragraph 0057-0060; 0067-0070; 0077-0080; 0087-0090
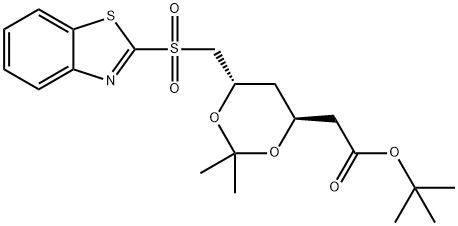
1054627-26-7
0 suppliers
inquiry
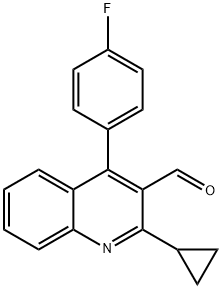
121660-37-5
176 suppliers
$9.00/250mg
![(4R,6S)-6-[(1E)-2-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester](/CAS/GIF/147489-06-3.gif)
147489-06-3
180 suppliers
$99.00/1g