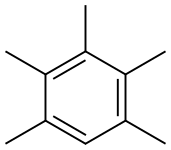
Pentamethylbenzene synthesis
- Product Name:Pentamethylbenzene
- CAS Number:700-12-9
- Molecular formula:C11H16
- Molecular Weight:148.24
Pentamethylbenzene has been observed as an intermediate in the formation of hexamethylbenzene from phenol and alkylation of durene or pentamethylbenzene has been reported as a suitable starting material for the synthesis of hexamethylbenzene.

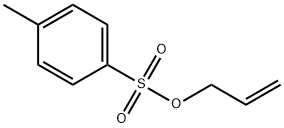
67-56-1
779 suppliers
$7.29/5ml-f

108-08-7
79 suppliers
$47.29/2g
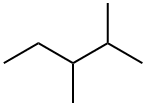
565-59-3
86 suppliers
$44.20/5g
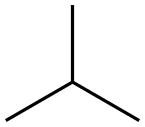
75-28-5
129 suppliers
$40.00/1g
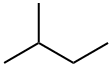
78-78-4
279 suppliers
$20.00/25ML
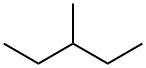
96-14-0
107 suppliers
$22.19/5ml
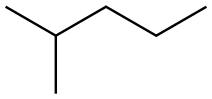
107-83-5
240 suppliers
$10.00/1g
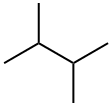
79-29-8
140 suppliers
$20.00/1g
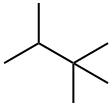
464-06-2
96 suppliers
$23.50/1g
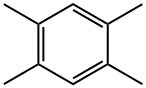
95-93-2
225 suppliers
$20.00/25g
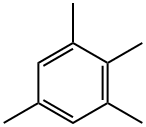
527-53-7
83 suppliers
$127.00/5mL

700-12-9
160 suppliers
$21.00/5g
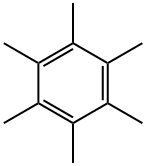
87-85-4
176 suppliers
$25.19/1g
Yield:464-06-2 16.7%
Reaction Conditions:
with isopropyl alcohol;indium (III) iodide at 200; for 2 - 3 h;
Steps:
39a; 39c
Mixtures of InI3 and methanol, in molar ratios varying from 1 :2 to 1 :4, along with a initiator (typically 2.5 mol% /-propanol) were heated in a closed vessel at 200 0C. Approximately two hours are required for complete conversion of methanol/DME to hydrocarbons and water. Increasing the relative amount of methanol inhibits reaction: at a molar ratio of 1 :5 only traces of triptane form under the above conditions. However, more than 5 equivalents of methanol per In can be converted as follows: 1 -2
References:
CALIFORNIA INSTITUTE OF TECHNOLOGY;BP CHEMICALS LIMITED WO2008/24896, 2008, A2 Location in patent:Page/Page column 19-20; 25-26
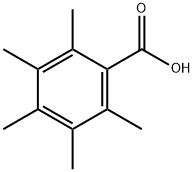
2243-32-5
27 suppliers
inquiry

700-12-9
160 suppliers
$21.00/5g
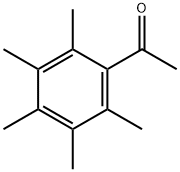
2040-01-9
37 suppliers
$460.00/250g

700-12-9
160 suppliers
$21.00/5g
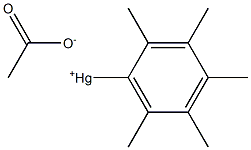
56457-41-1
0 suppliers
inquiry

700-12-9
160 suppliers
$21.00/5g