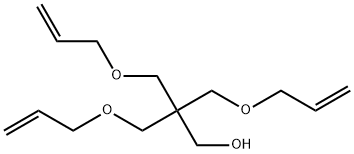
PENTAERYTHRITOL TRIALLYL ETHER synthesis
- Product Name:PENTAERYTHRITOL TRIALLYL ETHER
- CAS Number:1471-17-6
- Molecular formula:C14H24O4
- Molecular Weight:256.34
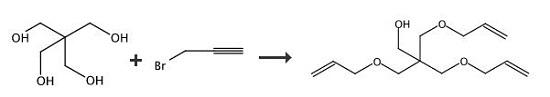
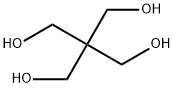
3-(Prop-2-ynyloxy)-2,2-bis((prop-2-ynyloxy)methyl)propan-1-ol (35). An aqueous solution of NaOH (40 wt %, 10 mL) was added to a solution of pentaerythritol (2.00 g, 14.7 mmol) in 15 mL of dimethylsulfoxide (DMSO). The solution was stirred at rt for 30 min. Propargyl bromide (97%, 9.8 mL, 110 mmol) was then added, and the solution was kept at rt for an additional 10 h. The reaction mixture was then poured into 150 mL of EtOAc and washed with H2O (50 mL × 2) as well as brine (50 mL). The organic layer was dried by Na2SO4 and filtered. The filtrate was concentrated, and the residue was purified by FC (EtOAc/hexane = 1/4). 2.29 g of yellowish oil 35 (yield: 62%). 1H NMR (200 MHz, CDCl3) δ: 4.13 (d, 6H, J = 2.4 Hz), 3.69 (d, 2H, J = 6.4 Hz), 3.56 (s, 6H), 2.43 (t, 3H, J = 2.4 Hz).
Yield:1471-17-6 92%
Reaction Conditions:
with allyl alcohol;sodium hydroxide in water at 90 - 1000; under 760.051 Torr; for 8.5 h;Dean-Stark;
Steps:
1; 1; 2; 2 Example 2
The reactor equipped with a condenser is the same as that of Example 1, and 72 grams (2 moles) of pentaerythritol is added.Then 167 grams of sodium hydroxide aqueous solution (2 moles of sodium hydroxide) with a 48% mass concentration was added, and then 10 grams of allyl alcohol was added.The temperature rises to dissolve the pentaerythritol.Under normal pressure and temperature conditions of 1000°C, 3-chloropropene was added in the same way as in Example 1.The cumulative volume is 16 ml at 1 hour, and 50 ml after 2 hours.Then it was cooled to 400°C, the same as in Example 1, and 417 grams of sodium hydroxide aqueous solution of 48% mass concentration (sodium hydroxide 5 moles,After 7 moles in total), the temperature is increased again.The reaction temperature is controlled at 90-1000°C by continuous dripping of chloropropene.The water steamed into the water separator together with the 3-chloropropene should be gradually transferred out.520 ml (6.4 moles, 7 moles in total) of 3-chloropropene was added dropwise within 5 hours from the second start of the reaction.After the end, reflux for another 30 minutes and cool.Add 1200 milliliters of water to the above reaction solution to dissolve the generated water and separate the oil layer and water layer.Then, the low boilers were removed in the same way as in Example 1, and 468 grams of pentaerythritol allyl ether containing 0.5% water was obtained.Its composition is zero monoether, 11.5% diether, 81% triether, and 7.5% tetraether. The yield of pentaerythritol allyl ether based on pentaerythritol was 92%.
References:
CN112645804,2021,A Location in patent:Paragraph 0037-0047
115-77-5
6 suppliers
$10.00/10g
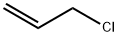
107-05-1
411 suppliers
$16.80/100ML
![3,3'-[[2,2-bis[(allyloxy)methyl]-1,3-propanediyl]bis(oxy)]dipropene](/CAS/GIF/1471-18-7.gif)
1471-18-7
7 suppliers
inquiry

1471-17-6
96 suppliers
inquiry

115-77-5
6 suppliers
$10.00/10g
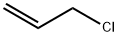
107-05-1
411 suppliers
$16.80/100ML
![3,3'-[[2,2-bis[(allyloxy)methyl]-1,3-propanediyl]bis(oxy)]dipropene](/CAS/GIF/1471-18-7.gif)
1471-18-7
7 suppliers
inquiry
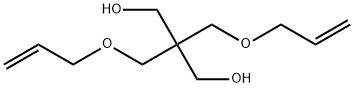
2590-16-1
4 suppliers
inquiry

1471-17-6
96 suppliers
inquiry
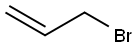
106-95-6
427 suppliers
$10.00/5g

1471-17-6
96 suppliers
inquiry