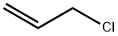
Allyl chloride synthesis
- Product Name:Allyl chloride
- CAS Number:107-05-1
- Molecular formula:C3H5Cl
- Molecular Weight:76.52

107-18-6
2 suppliers
$24.60/100ml
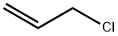
107-05-1
411 suppliers
$16.80/100ML
Yield:107-05-1 82%
Reaction Conditions:
with benzoyl chloride;N,N-dimethyl-formamide at 0 - 20; for 5.5 h;
Steps:
4.4.3.8 Synthesis of Allyl Chloride (216)
General procedure: 2 1 According to general procedure Ill (chapter 2.1.3) a 100 mL flask with a strong stir bar3-Clwas charged with allyl alcohol 116 (13.8 mL, 11.73 g, 200 mmol, 1.0 equiv) and DMF216 (4.6 mL, 4.39 g, 60.0 mmol, 30 mol%). The reaction solution was cooled in an ice bathand benzoyl chloride (24.6 mL, 29.82 g, 210 mmol, 1.05 equiv) was added slowly through a dropping funnel within 1.5 h. Then the cooling bath was removed and the mixture was allowed to stir for 4 h atroom temperature, whereby benzoic acid started to precipitate (after 1 h). ‘H-N MR after 3.5 h ofstirring at ambient temperature proved full consumption of the starting material 116. The whitereaction suspension was next subjected to distillation at 1 atm through a Claisen distillation bridgewith a 20 cm water cooler to yield allyl chloride 216 as a colorless liquid with bp. of 41-42 °C (12.45 g,162.3 mmol, 82%). Thereby the distillation apparatus was equipped with a KOH-drying tube and thecollecting flask was cooled in an ice bath. To achieve complete separation of the product 216 from thereaction mixture the oil bath temperature had to be raised from 90 to 180 °C.M (C3H5CI) = 76.53 g/mol
References:
WO2016/202894,2016,A1 Location in patent:Page/Page column 53; 109; 110
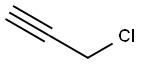
624-65-7
374 suppliers
$25.00/5mL
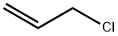
107-05-1
411 suppliers
$16.80/100ML
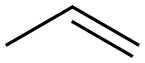
187737-37-7
1 suppliers
inquiry
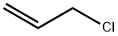
107-05-1
411 suppliers
$16.80/100ML

106-89-8
753 suppliers
$9.00/10g
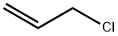
107-05-1
411 suppliers
$16.80/100ML

187737-37-7
1 suppliers
inquiry

592-42-7
176 suppliers
$22.00/25mL
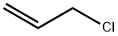
107-05-1
411 suppliers
$16.80/100ML