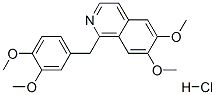
Papaverine hydrochloride synthesis
- Product Name:Papaverine hydrochloride
- CAS Number:61-25-6
- Molecular formula:C20H22ClNO4
- Molecular Weight:375.85
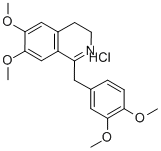
5884-22-0
69 suppliers
inquiry

61-25-6
224 suppliers
$10.00/500mg
Yield:61-25-6 89.1%
Reaction Conditions:
Stage #1:1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-3.4-dihydroisoquinoline hydrochloride with sodium hydroxide in water
Stage #2: in tetralin at 175;
Stage #3: with hydrogenchloride in methanol;water
Steps:
1.2; 2.2; 3.2
(2) Dissolve the 6,7,3',4'-tetramethoxy-1-benzyl-dihydro-isoquinoline hydrochloride (average water content: 8-15%) obtained in the previous step in 600 mL In hot waterAdd concentrated sodium hydroxide solution to make the solution phenolphthalein indicator alkaline,Filter out the precipitated 6,7,3',4'-tetramethoxy-1-benzyl-dihydro-isoquinoline (substance C), and carefully wash with water to remove sodium hydroxide;Dissolve the wet product in 330gTetrahydronaphthalene and 85g phenylethyl ether, at 175Dehydrogenation reaction in the presence of 12g Raney nickel catalyst;After the dehydrogenation is complete,The tetralin reaction mixture is filtered directly from the Raney nickel catalyst to20% hydrochloric acid aqueous solution and methanol 1:1 mixture; filter out the precipitate,In an inert gas environment (nitrogen) from ethanol-water-isobutanol solution (1:1:0.3)Medium-recrystallized, almost white6,7,3',4'-Tetramethoxy-1-benzylisoquinoline hydrochloride(Ie papaverine hydrochloride) (the yield of this step is 89.1%).
References:
Shan Northeast Da High-tech Huatai Pharmaceutical Co., Ltd.;Zhang Genglun;Zhou Hongjuan;Zhang Shuang;Xia Xiaoli CN111848512, 2020, A Location in patent:Paragraph 0251; 0253-0255; 0257-0258; 0259; 0261-0262
58-74-2
4 suppliers
$75.00/10mg

61-25-6
224 suppliers
$10.00/500mg
93-40-3
470 suppliers
$10.00/5g

61-25-6
224 suppliers
$10.00/500mg
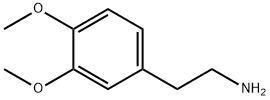
120-20-7
560 suppliers
$5.00/5 g

61-25-6
224 suppliers
$10.00/500mg
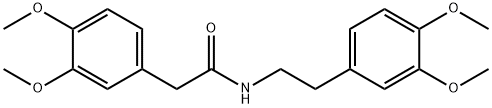
139-76-4
33 suppliers
inquiry

61-25-6
224 suppliers
$10.00/500mg