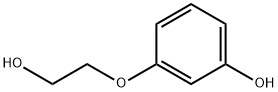
O-HYDROXYETHYLRESORCINOL synthesis
- Product Name:O-HYDROXYETHYLRESORCINOL
- CAS Number:49650-88-6
- Molecular formula:C8H10O3
- Molecular Weight:154.16
![Acetic acid, 2-[3-(acetyloxy)phenoxy]-, ethyl ester](/CAS/20210305/GIF/474010-11-2.gif)
474010-11-2
0 suppliers
inquiry

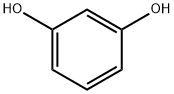
49650-88-6
29 suppliers
inquiry
Yield:49650-88-6 100%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20; for 2 h;
Steps:
38-1
To a solution of 3-acetoxyphenol (3.04g, 20ml) in dimethylformamide were added calcium carbonate (2.76g, 20mmol) and ethyl bromoacetate (2.2ml, 20mmol), and the mixture was stirred for 2 hours at room temperature. The reaction was quenched by water and the mixture was extracted with ethyl acetate. The organic layer was washed with water, 10% aqueous citric acid solution, an aqueous saturated sodium hydrogencarbonate solution and an aqueous saturated sodium chloride solution, and dried over sodium sulfate. The solvent was removed under reduced pressure and the residue was purified with silica gel chromatography (hexane:ethyl acetate=10:1) to give ethyl [3-(acetyloxy)phenoxy]acetate (3.30g, 69%). To a solution of lithium aluminum hydride (455mg, 12mmol) in tetrahydrofuren was dropped ethyl [3-(acetyloxy)phenoxy]acetate (1.19g, 5mmol) in tetrahydrofuran (20ml) at room temperature, and the mixture was stirred for 2 hours at room temperature. The reaction was quenched by 10% hydrochloric acid and the mixture was extracted with ethyl acetate. The organic layer was washed with water, 10% hydrochloric acid, an aqueous saturated sodium hydrogencarbonate solution and an aqueous saturated sodium chloride solution. The organic layer was dried over sodium sulfate. The solvent was removed under reduced pressure to give 3-(2-hydroxyethoxy)phenol (864mg, quantitatively). To a solution of 3-(2-hydroxyethoxy)phenol (864mg, 5mmol) in tetrahydrofuran (20ml) were added potassium carbonate (967mg, 7mmol) and t-butyl bromoacetate (0.89ml, 6mmol), and the mixture was stirred for 2 hours at room temperature. The reaction was quenched by 10% aqueous citric acid solution and the mixture was extracted with ethyl acetate. The organic layer was washed with water, 10% aqueous citric acid solution, an aqueous saturated sodium hydrogencarbonate solution and an aqueous saturated sodium chloride solution. The organic layer was dried over sodium sulfate. The solvent was removed under reduced pressure and the residue was purified with silica gel chromatography (hexane:ethyl acetate=3:1) to give the subject compound (3.30g, 69%).1H NMR (CDCl3, 400 MHz) δ 7.18 (t, 1 H, J = 8.1 Hz), 6.56 (dd, 1 H, J = 8.1 Hz, 1.7 Hz), 6.46 - 6.52 (m, 2 H), 4.49 (s, 2H), 4.06 (t, 2 H, J = 4.5 Hz), 3.92 - 3.97 (m, 2 H), 2.04 (t, 2 H, J = 6.2 Hz), 1.49 (s, 9 H).
References:
EP1386913,2004,A1 Location in patent:Page 92
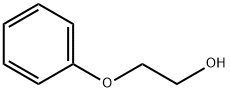
122-99-6
758 suppliers
$8.00/100g
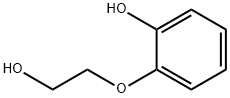
4792-78-3
71 suppliers
$60.00/50g

49650-88-6
29 suppliers
inquiry
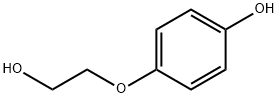
13427-53-7
9 suppliers
inquiry