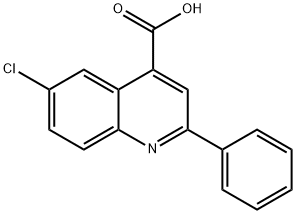
NSC42124 synthesis
- Product Name:NSC42124
- CAS Number:6633-62-1
- Molecular formula:C16H10ClNO2
- Molecular Weight:283.71
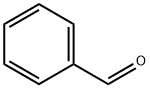
100-52-7
950 suppliers
$21.30/2g
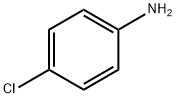
106-47-8
471 suppliers
$5.00/5G
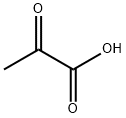
127-17-3
529 suppliers
$5.00/10g

6633-62-1
24 suppliers
$75.00/500mg
Yield:6633-62-1 79%
Reaction Conditions:
Stage #1: benzaldehyde;4-chloro-anilinewith borane-THF in acetonitrile at 65; for 0.166667 h;
Stage #2: 2-oxo-propionic acid in acetonitrile; for 24 h;
Steps:
A5 Example A5
Production of 6-chloro-2-phenylquinoline-4-carboxylic acid
To a solution of 4-chloroaniline (1.30 g, 10.2 mmol) in acetonitrile (9.5 mL), benzaldehyde (1.21 g, 11.4 mmol) and boron trifluoride-tetrahydrofuran complex (313 μL, 2.84 mmol) were added, and the mixture was heated to 65° C. 10 minutes later, a solution of pyruvic acid (0.500 g, 5.68 mmol) in acetonitrile (5.7 mL) was added dropwise thereto over 3 hours. 21 hours later, the reaction solution was cooled to room temperature, and saturated aqueous sodium chloride solution was added thereto, followed by extraction with tetrahydrofuran.
The organic layer was washed with saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, and then filtered, and the solvent was distilled off under reduced pressure.
The obtained residue was suspended and washed in chloroform and dried under reduced pressure to obtain the title compound (1.27 g, yield: 79%) as an ocher solid.
1H-NMR (400 MHz, DMSO-D6) δ: 14.1 (1H, s), 8.79 (1H, d, J=2.4 Hz), 8.56 (1H, s), 8.32-8.28 (2H, m), 8.19 (1H, d, J=9.2 Hz), 7.89 (1H, dd, J=9.2, 2.4 Hz), 7.60-7.55 (3H, m)
ESI-MS (m/z): 284, 286 (M+H)+
References:
US2022/169640,2022,A1 Location in patent:Paragraph 0152-0154
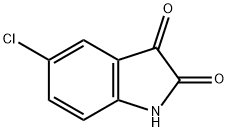
17630-76-1
284 suppliers
$6.00/5g
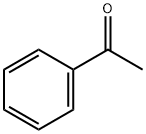
98-86-2
772 suppliers
$9.00/5g

6633-62-1
24 suppliers
$75.00/500mg

106-47-8
471 suppliers
$5.00/5G

127-17-3
529 suppliers
$5.00/10g

6633-62-1
24 suppliers
$75.00/500mg

106-47-8
471 suppliers
$5.00/5G

6633-62-1
24 suppliers
$75.00/500mg