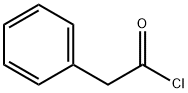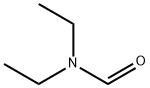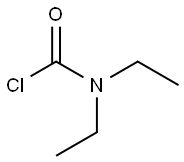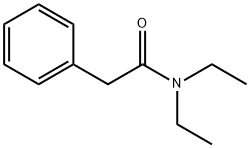
N,N-DIETHYL-2-PHENYLACETAMIDE synthesis
- Product Name:N,N-DIETHYL-2-PHENYLACETAMIDE
- CAS Number:2431-96-1
- Molecular formula:C12H17NO
- Molecular Weight:191.27
Yield: 93%
Reaction Conditions:
with silver(I) acetate;sodium acetate in diethyl ether at 20; for 0.25 h;Inert atmosphere;Darkness;
Steps:
The following experimental procedure reported for the silver acetate-assisted synthesis of N,N-diethylbenzamide (1) is representative for the preparation of amides 2-17 and N-(4-nitrobenzenesulfonyl)-protected dipeptide methyl esters 18 and 19.
General procedure: N,N-Diethylamine (1mmol), silver acetate (1mmol) and sodium acetate (2mmol) were added to a solution of benzoyl chloride (1mmol) in diethyl ether (10mL). The heterogeneous mixture was magnetically stirred at room temperature for 15 minutes, preserving the reaction flask from exposure to light. After this time, a white precipitate was formed and TLC (diethyl ether/P.E. 70:30 v/v) showed the complete conversion of the starting chloride. The mixture was paper filtered, washed with 1N aqueous HCl (3×5mL), 1N aqueous NaOH (3×5mL) and once with brine (5mL). The ethereal layers were dried (Na2SO4) and evaporated to dryness under reduced pressure conditions. N,N-Diethylbenzamide (1) was recovered as a viscous colorless oil without need for chromatography. Yield: 92%; TLC: Rf=0.77; 1H NMR (300MHz, CDCl3) δ: 7.31-7.40 (m, 5H, ArH), 3.52 (m, 2H, CH2), 3.23 (m, 2H, CH2), 1.25 (m, 3H, CH3) 1.10 (m, 3H, CH3); 13C NMR (75MHz, CDCl3) δ: 171.3, 137.2, 129.1, 128.4, 126.2, 43.3, 39.2, 14.2, 12.9. MS (EI, 70eV) m/z (% rel.): 177 [M+], 176 (49), 162 (8), 148 (16), 134 (12), 105 (100), 77 (48), 51 (21). Anal. Calcd for C11H15NO: , 74.54; , 8.53; N, 7.90. Found: , 74.65; , 8.51; N, 7.88.
References:
Leggio;Belsito;Di Gioia;Leotta;Romio;Siciliano;Liguori [Tetrahedron Letters,2014,vol. 56,# 1,p. 199 - 202]
201230-82-2
1 suppliers
inquiry
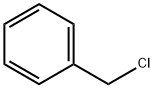
100-44-7
643 suppliers
$13.50/250G

109-89-7
467 suppliers
$10.00/5g

2431-96-1
72 suppliers
$28.00/1g