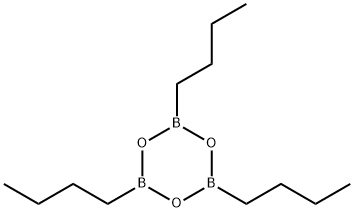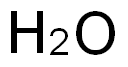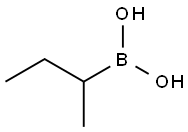
n-Butylboronic acid synthesis
- Product Name:n-Butylboronic acid
- CAS Number:4426-47-5
- Molecular formula:C4H11BO2
- Molecular Weight:101.94

693-03-8
60 suppliers
$153.00/50mL
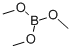
121-43-7
358 suppliers
$13.00/25mL

4426-47-5
344 suppliers
$6.00/1g
Yield:4426-47-5 74.88%
Reaction Conditions:
with hydrogenchloride in water
Steps:
VII 1.2 Preparation of Butyl Boronic Acid 18
1.2 Preparation of Butyl Boronic Acid 18 To a 1-L, three-necked, round bottomed flask equipped with a magnetic stirrer and thermometer, was added 480 ml of ether, followed by 20 ml (176 mmol) of trimethylborate. The clear solution was cooled to -75° C. (internal temperature) and vigorously stirred, then 90 ml (176 mmol) of 1.95 M solution of butylmagnesium bromide in ether was added dropwise via cannula at such a rate that the internal temperature did not exceed -65° C. After the addition was completed, the resulting white slurry was stirred for an additional 2 hours at -75° C. under nitrogen. The cooling bath was then removed and the reaction mixture was allowed to warm to room temperature (between 1h and 2h are needed). Hydrolysis was carried out by the dropwise addition of 200 ml of a 10% aqueous solution of hydrochloric acid. The white precipitate was dissolved and the resulting clear biphasic mixture was stirred for 15 min, after which time, the two layers were separated. The aqueous layer was extracted with ether (2*100 ml), and the combined extracts dried over magnesium sulfate. After concentration of the ethereal solution under reduced pressure, the residual white solid was purified by recrystallization as follows: after dissolution in hot water (50 ml), the resulting biphasic solution was cooled to 0° C. to induce recrystallization of the boronic acid. The solid was collected on a medium fritted disk funnel and washed with 100 ml of hexanes and placed under vacuum for 60 min. A quantity of 13.6 g of the boronic acid 18 was produced as a white solid. Yield: 74.88%; m.p.=94-96° C. (lit. m.p.=95-97° C.) (Charette, A. B.; Juteau, H.; Lebel, H.; and Molinaro C. J. Am. Chem. Soc. 1998, 120, 11943-11952); Characterization: Nmr (1H).
References:
Theratechnologies Inc. US6458764, 2002, B1
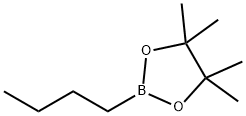
69190-62-1
92 suppliers
$21.00/5g

4426-47-5
344 suppliers
$6.00/1g

109-65-9
548 suppliers
$10.00/10g

4426-47-5
344 suppliers
$6.00/1g

109-65-9
548 suppliers
$10.00/10g
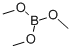
121-43-7
358 suppliers
$13.00/25mL

4426-47-5
344 suppliers
$6.00/1g