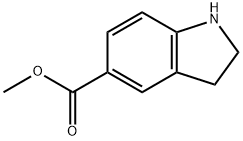
methyl indoline-5-carboxylate synthesis
- Product Name:methyl indoline-5-carboxylate
- CAS Number:141452-01-9
- Molecular formula:C10H11NO2
- Molecular Weight:177.2
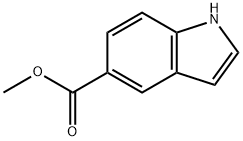
1011-65-0
277 suppliers
$6.00/250mg

141452-01-9
105 suppliers
$16.00/100mg
Yield: 99%
Reaction Conditions:
with sodium cyanoborohydride;acetic acid at 0 - 20; for 1.08333 h;
Steps:
19.A
To a solution of lH-Indole-5-carboxylic acid methyl ester (1 g, 5.71 mmol) in 10 mL of acetic acid at 0 0C, was added sodium cyanoborohydride (1.08 g, 17.18 mmol) over 5 minutes. The mixture was stirred at room temperature for Ih. Water (3 mL) was added and all the solvents were removed under vacuum. The residue was dissolved in ethyl acetate (150 mL) and saturated NaHCCh (150 mL). The layers were separated and the aqueous layer was extracted with ethyl acetate (3 x 75 mL). The combined extracts were washed with brine (150 mL), dried, filtered and evaporated. The residue was purified by flash chromatography (0-50% ethyl acetate in hexane + 0.1% triethylamine) to afford 0.99 g (99%) of 19a.
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH;NEUROCRINE BIOSCIENCES, INC.;TRAN, Joe, A.;CHEN, Chen WO2010/149685, 2010, A1 Location in patent:Page/Page column 51
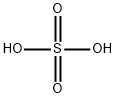
7664-93-9
6 suppliers
$21.64/1003

1011-65-0
277 suppliers
$6.00/250mg

141452-01-9
105 suppliers
$16.00/100mg

67-56-1
752 suppliers
$7.29/5ml-f
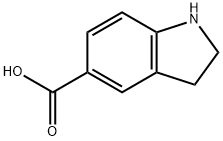
15861-30-0
98 suppliers
$39.00/100mg

141452-01-9
105 suppliers
$16.00/100mg
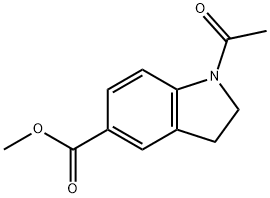
153497-12-2
0 suppliers
inquiry

141452-01-9
105 suppliers
$16.00/100mg
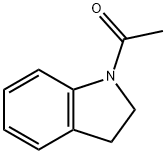
16078-30-1
191 suppliers
$9.00/5g

141452-01-9
105 suppliers
$16.00/100mg