
methoxymethanol synthesis
- Product Name:methoxymethanol
- CAS Number:4461-52-3
- Molecular formula:C2H6O2
- Molecular Weight:62.07
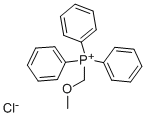
4009-98-7
353 suppliers
$5.00/10g
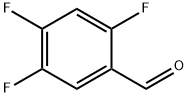
165047-24-5
237 suppliers
$6.00/1g

4461-52-3
3 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:(methoxymethyl)triphenylphosphonium chloride with potassium tert-butylate in tetrahydrofuran at 20; for 1 h;
Stage #2:2,4,5-trifluorobenzaldehyde in tetrahydrofuran at 20; for 48 h;optical yield given as %de;Wittig reaction;
Steps:
tert-Butyl (E)-4-(2',4',5'-trifluorophenyl)but-2-enoate 6
Step 1: KOtBu (17.5 g, 156.3 mmol) was added to methoxymethyltriphenylphosphonium chloride (53.6 g, 156 mmol) in THF (300 mL) at rt and the resultant mixture was stirred for 1 h. 2,4,5-Triflurobenzaldehyde 4 (5.0 g, 31.3 mmol) was added dropwise at rt and stirring was continued at rt for 48 h. The suspension was filtered and the filter cake was washed with EtOAc (3 45 mL). The combined organics were sequentially washed with satd aq NH4Cl (500 mL), brine (300 mL), then dried and concentrated in vacuo. Purification via flash column chromatography (eluent hexane/EtOAc, 20:1) gave a 1:1 mixture of 5 (55:45 dr) and a by-product (which was tentatively assigned as methoxymethanol) as a light yellow oil (7.19 g). Data for (E)-5: δH (400 MHz, CDCl3) 3.72 (3H, s, OMe), 5.80 (1H, d, J 13.0, HC=CH), 6.926.96 (1H, m, Ar), 7.027.08 (1H, m, Ar), 7.15 (1H, d, J 13.0, HC=CH). Data for (Z)-5: δH (400 MHz, CDCl3) 3.82 (3H, s, OMe), 5.44 (1H, d, J 7.0, HC=CH), 6.26 (1H, d, J 7.0, HC=CH), 7.027.08 (1H, m, Ar), 7.747.79 (1H, m, Ar). Data for methoxymethanol:2 δH (400 MHz, CDCl3) 3.41 (3H, s, OMe), 4.46 (2H, s, CH2).
References:
Davies, Stephen G.;Fletcher, Ai M.;Lv, Linlu;Roberts, Paul M.;Thomson, James E. [Tetrahedron Letters,2012,vol. 53,# 24,p. 3052 - 3055] Location in patent:supporting information; experimental part

67-56-1
756 suppliers
$7.29/5ml-f
50-00-0
867 suppliers
$10.00/25g

4461-52-3
3 suppliers
inquiry
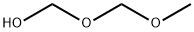
19942-08-6
0 suppliers
inquiry

67-56-1
756 suppliers
$7.29/5ml-f

4461-52-3
3 suppliers
inquiry
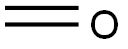
50-00-0
867 suppliers
$10.00/25g

4461-52-3
3 suppliers
inquiry